All published articles of this journal are available on ScienceDirect.
MCP1, CRP and Procalcitonin as Novel Diagnostic Markers in Cirrhotic Patients with Spontaneous Bacterial Peritonitis
Abstract
Background & Aims:
The aim of the study was to evaluate serum c-reactive protein (CRP), ascitic procalcitonin (PCT) and monocyte chemotactic protein-1 (MCP-1) in the diagnosis of spontaneous bacterial peritonitis (SBP) in cirrhotic patients.
Methods:
A cross-sectional analytic study that included 199 patients with decompensated cirrhosis (101 with SBP and 98 without SBP). Patients were classified according to Child-Pugh criteria. Ascitic PCT and MCP-1 were measured by enzyme-linked immunosorbent assay. Serum CRP, liver and renal functions were assessed.
Results:
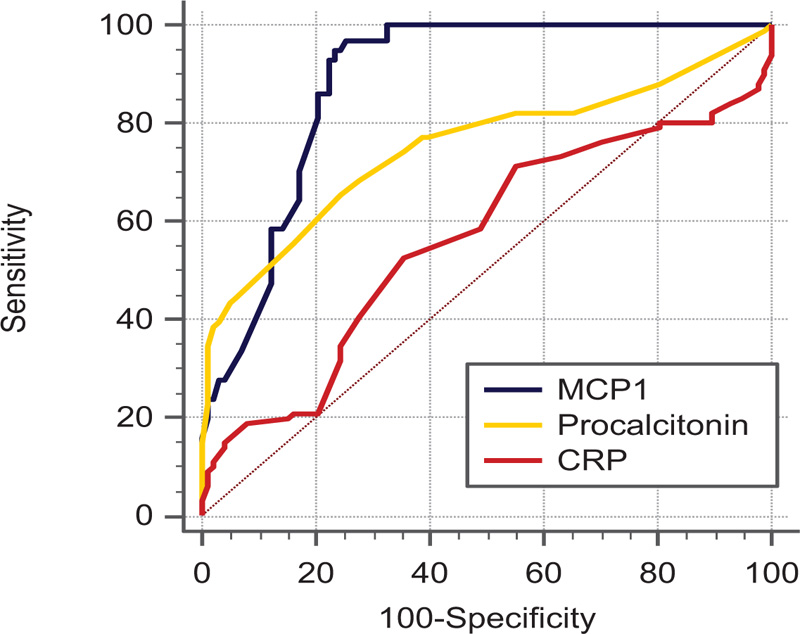
Three markers are significantly elevated in SBP patients than those without ascites. Using the ROC curve at AUC 0.883 and a cut-off value of >186 ng/ml, the diagnostic performance of ascitic MCP-1 level was higher than CRP (AUC 0.562) and ascitic fluid procalcitonin (AUC 0.751) in the diagnosis of SBP. The sensitivity and specificity were 86.15% and 79.59% at the cutoff of 186 ng/ml for MCP-1, 65.4 and 75.5 at ≥ 1 ng/ml for PCT, and 52.5 and 64.3, respectively for at 11.2 mg/dl CRP.
Conclusion:
Ascitic MCP-1 has a better diagnostic value with higher sensitivity and specificity in diagnosis SBP compared to CRP and procalcitonin which has higher diagnostic accuracy than CRP. Further studies with a large number will be necessary to evaluate the usefulness of these markers in diagnosis, follow-up and relation to morbidity and mortality of SBP patients.
1. INTRODUCTION
Spontaneous bacterial peritonitis (SBP) is one of the major complications and the most frequent bacterial infection in patients with decompensated cirrhosis and is associated with high morbidity and mortality [1]. SBP has been defined as a bacterial infection of ascitic fluid without any intra-abdominal surgically treatable source of infection [2]. One-half of the episodes of SBP are acquired during hospitalization [3].
The early diagnosis of SBP is based on a high index of suspicion and may be challenged by the fact that typical features, like fever and abdominal pain or leukocytosis, are frequently absent [4]. Early suspicion and identification of SBP is a key issue in the appropriate management of SBP and may provide the best opportunity for a better outcome and improving survival, especially for those at risk of poor prognosis [5, 6]. The diagnosis of SBP is based on polymorphonuclear leukocyte count ≥250/mm3 in ascitic fluid, with or without positive ascitic culture, in the absence of other causes of peritonitis [6-9]. Also, Ascites culture is essential to guide antibiotic therapy, negative culture occurs nearly in 60% of patients with clinical pictures suggestive of SBP [10, 11]. In cases of emergency setting, ascitic fluid culture examination is time-consuming and sometimes not available in the emergency setting [12, 13]. Patients with bactericides (polymorphonuclear leukocyte count <250/mm3 but positive bacterial culture) with signs of infection or systemic inflammation should be treated with antibiotics [7]. There is a need for easy-to-apply, rapid and reliable markers to predict the diagnosis and prognosis of ascitic patients.
It is well known that C-reactive protein (CRP) and procalcitonin (PCT) increased rapidly in response to bacterial infection. They are sensitive diagnostic markers that can be used to predict diagnosis, monitor bacterial infections and guide the clinical use of antibiotics [14, 15].The acute phase reactant proteins, such as CRP and PCT have been investigated as tools for early diagnosis of SBP in various clinical conditions [16, 17]. Monocyte Chemotactic Protein-1(MCP-1) is one of the most potent chemokines for monocytes/macrophages and activated lymphocytes during infections [18]. In addition, several studies have shown that neutrophil infiltration is affected by MCP-1 [19]. This work aimed to evaluate CRP, ascitic fluid PCT and MCP-1 in ascitic patients with or without SBP.
2. MATERIALS AND METHODS
This study was an analytical cross-sectional study that included 199 patients with decompensated cirrhosis from the Tropical Medicine Department, Tanta Faculty of Medicine during the period from April 2021 to October 2021. The institutional ethical committee approved the study of Tanta University Faculty of Medicine with approval number 30271\04\015. Informed consent was obtained from all participants of this study. The patients were divided into two groups. Group (A) included 101 patients with SBP based on the clinical picture and ascitic fluid polymorphonuclear leukocyte count ≥250/mm3 in whom antibiotic treatment for SBP had not yet been started. Group (B) included 98 patients without SBP with ascitic fluid polymorphonuclear leukocyte count <250/mm3 without evidence of infection.
Patients with ascites because of any other cause other than liver diseases (malignancy, tuberculosis) were excluded based on history and laboratory and radiological findings. Ascitic patients receiving antibiotics 2 weeks before diagnosis or patients on prophylaxis of SBP as it could alter the result were excluded. Also, infections other than SBP (gastroenteritis, meningitis, skin, chest, biliary tract, urinary tract, and dental infections) were excluded.
All participants were subjected to the following: Full medical history and complete physical examination. Laboratory investigations were carried out, including complete blood picture, Aspartate aminotransaminase (AST), (ALT) alanine aminotransaminase, bilirubin, serum albumin, creatinine and Glucose were done by automatic chemistry auto analyzer (Cobas 6000). Prothrombin time concentration and international normalized ratio were also determined. Complete blood picture was done by pheonix. Diagnosis of spontaneous bacterial peritonitis was made by a Blood count analyzer (count of polymorph nuclear leucocytes ≥ 250mm3 considered as spontaneous bacterial peritonitis). Measurement of procalcitonin, Alpha feto protein were done for every participant in this study by statfax 2100 (Eliza reader provided from Gama trade company in Giza). Monocyte Chemotactic protein-1(MCP-1) levels were done by ELISA according to manufacture instructions (Manufactured and Distributed by: USA R&D Systems, Inc, Catalog Number DCP00 provided from clinilab company in Maadi). Pelvi-abdominal ultrasound (Using Toshiba, made in Japan, Model SSA-660A) was performed on all patients.
2.1. Statistical Analysis
2.2 Statistical tests were performed using IBM SPSS software (Version 21.0, Armonk, NY). Qualitative data were expressed as number and percentages and quantitative variables were expressed as the mean ± standard deviation (mean ±SD) for parametric data while nonparametric data were expressed as (median and IQR). The Mann–Whitney test was used to compare non-normally distributed quantitative data, and χ2 test was used to evaluate qualitative data. The receiver operating characteristic (ROC) curves were generated to test the accuracies and cut-off values for different markers and determine optimum cut-off values by maximizing sensitivity and specificity. A level for statistical significance was significant at p < 0.05 (Fig. 1 and Table 1).
| Variables | Ascites without SBP N=98 |
Ascites with SBP N=101 |
Test | P | |
|---|---|---|---|---|---|
| Age | Mean ±SD | 54.01±12.28 | 56.07±9.51 | -1.325 | 0.187 |
| Range | (35-77) | (28-80) | |||
| Gender N (%) |
Male | 74 (75.50) | 76 (75.20) | 0.002 | 0.966 |
| Female | 24 (24.50) | 25 (24.80) | |||
| Ascites N (%) |
Mild | 4 (4.08) | 0 (0.00) | 5.033 | 0.0807 |
| Moderate | 46 (46.94) | 56 (55.45) | |||
| Severe | 48 (48.98) | 45 (44.55) | |||
| Child Score N (%) |
B | 66 (67.30) | 61 (60.40) | 1.36 | 0.3039 |
| C | 32 (32.70) | 40 (39.60) | |||
| Variables | Ascites without SBP N=98 |
Ascites with SBP N=101 |
Test | P | |
|---|---|---|---|---|---|
| Hb (gm/dl) |
Range | 7-14 | 6.5-15 | 0.385 | 0.7004 |
| Median | 10.5 | 10.3 | |||
| IQR | 9.0-11.0 | 9.3-11.2 | |||
| WBCS (103/µl) |
Range | 2.5-12.0 | 4.5-17.0 | 6.973 | < 0.0001* |
| Median | 7.50 | 8.80 | |||
| IQR | 6.2-8.6 | 8.5-9.0 | |||
| PLT (103/µl) |
Range | 19-450 | 13-473 | 1.944 | 0.0518 |
| Median | 105 | 110 | |||
| IQR | 88-135 | 93-141 | |||
| RBS (mg/dl) |
Range | 85-170 | 80-180 | 1.848 | 0.0647 |
| Median | 99 | 102 | |||
| IQR | 96-114 | 99-120 | |||
| Creatinine (mg/dl) |
Range | 0.7-1.2 | 0.7-2.8 | 9.15 | < 0.0001* |
| Median | 0.95 | 1.6 | |||
| IQR | 0.8-1.1 | 1.33 | |||
| Total Bilirubin (mg/dl) |
Range | 0.4-12 | 0.4-22 | 1.123 | 1.962 |
| Median | 1.7 | 1.8 | |||
| IQR | 1.2-2.3 | 1.5-2.7 | |||
| Albumin (g/l) |
Range | 1.4-3.4 | 2-4 | 1.316 | 1.881 |
| Median | 3.1 | 3 | |||
| IQR | 2.50-3.1 | 2.4-3.1 | |||
| INR | Range | 1.1-2.6 | 1-2.16 | 1.820 | 0.688 |
| Median | 1.45 | 1.49 | |||
| IQR | 1.0-1.70 | 1.26-1.70 | |||
| ALT (u/l) |
Range | 36-180 | 20-210 | 0.849 | 0.396 |
| Median | 57 | 59 | |||
| IQR | 50-70 | 43-78 | |||
| AST (u/l) |
Range | 30-210 | 40-340 | 1.504 | 0.133 |
| Median | 80 | 88 | |||
| IQR | 70-90 | 76-106 | |||
| AFP (ng/ml) |
Range | 2.9-195 | 2.0-651 | 1.472 | 0.110 |
| Median | 8 | 16 | |||
| IQR | 5.0 23.0 | 8.50-27.0 | |||
| CRP (mg/l) |
Range | 1.7-13 | 1.0-65 | 3.110 | 0.041* |
| Median | 7 | 12 | |||
| IQR | 4.0-9.0 | 8.0-15.0 | |||
| PMNL (cell/mm3) | Range | 60-230 | 253-7000 | 11.943 | < 0.0001* |
| Median | 121 | 600 | |||
| IQR | 110-140 | 450-3400 | |||
| Procalcitonin (ng/ml) |
Range | 0.07-2.30 | 0.07-2.90 | 6.133 | < 0.0001* |
| Median | 0.40 | 1.50 | |||
| IQR | 0.2-1.0 | 0.70-2.20 | |||
| MCP1 (ng/ml) |
Range | 121-490 | 160-950 | 9.342 | < 0.0001* |
| Median | 150 | 210 | |||
| IQR | 134-167 | 190-300 | |||
| Parameter | AUC | 95% CI | Cut-off | Sensitivity | Specificity | PPV | NPV | P-Value |
|---|---|---|---|---|---|---|---|---|
| MCP-1 | 0.883 | 0.830 to 0.924 | >186 | 86.15 | 79.59 | 31.9 | 98.1 | <0.0001* |
| Procalcitonin | 0.751 | 0.685 to 0.809 | >1 | 65.35 | 75.51 | 22.9 | 95.1 | <0.0001* |
| CRP | 0.562 | 0.490 to 0.632 | >11.2 | 52.48 | 64.29 | 14.0 | 92.4 | 0.1352 |
| P value from pairwise comparisons | MCP1 vs Procalcitonin =0.019 MCP1 vs CRP =<0.0001 Procalcitonin vs CRP=0.0006 |
|||||||
| Parameter | Rho | 95% CI | P |
|---|---|---|---|
| MCP-1 | 0.940 | 0.921 to 0.954 | P<0.0001 |
| Procalcitonin | 0.727 | 0.655 to 0.787 | P<0.0001 |
| CRP | 0.310 | 0.179 to 0.431 | P<0.0001 |
3. RESULTS
A total of 199 decompensated chronic liver patients with ascites were enrolled in our study. 101out of 199 patients confirmed to have SBP with PMNL more than 250/mm3 while 98 patients were presented with sterile non-SBP ascites. No difference between the two studied groups regarding age and sex. Male patients were more common than females in both groups (nearly 75%) and the mean age was 54 years in the non-SBP group while 56 years in SBP patients. Most of the patients were presented with moderate to marked ascites without difference between the studied patients and their child score classification was (B > C) (Table 1). No statistically significant difference between SBP and non-SBP patients regarding hemoglobin level, platelet count, random blood sugar, serum albumin, total bilirubin, INR and AFP. The Median white blood cell count was higher in patients with SBP than non-SBP with a statistically significant difference (p-value < 0.0001) (Table 2). Serum creatinine was elevated in SBP patients (median 1.6 mg/dl) than non-SBP with a statistically significant difference. Both procalcitonin and MCP-1 and Serum CRP were elevated in SBP patients with statistically significant difference. Using the ROC curve at AUC 0.883 and a cut-off value of >186 ng/ml, the diagnostic performance of ascitic MCP-1 level was higher than CRP (AUC 0.562) and ascitic fluid procalcitonin (AUC 0.751) in the diagnosis of SBP with a statistically significant difference between them (pairwise comparisons with p < 0.001) (Table 3). The sensitivity and specificity were 86.15% and 79.59% at the cutoff of 186 ng/ml for MCP-1, 65.4 and 75.5 at ≥ 1 ng/ml for PCT, and 52.5 and 64.3, at 11.2 mg/dl for CRP (Table 3). There was with a strong positive correlation between MCP-1 and PMNL(Rho=0.94) followed by ascitic fluid procalcitonin then CRP (Table 4).
4. DISCUSSION
SBP is a life-threatening complication in advanced cirrhosis with ascites and is associated with high mortality especially with late or misdiagnosis [20-46]. The gold standard in the diagnosis of SBP still depends on PMNL in ascitic fluid of ≥ 250 cells/mm3 in the absence of intra-abdominal infection with or without a positive ascitic fluid culture [21]. Many markers in serum or ascitic fluid have been investigated for the diagnosis of SBP and their diagnostic value is still limited and large-scale studies are needed, such as tumor necrosis factor-α, interleukin-6, microRNA-155, procalcitonin, and amyloid A, C-reactive protein, CD64 and neutrophil-to-lymphocyte ratio [13, 22-24]. In this study, we evaluated different three markers (serum CRP, ascitic procalcitonin and MCP-1) in the diagnosis of SBP. The higher sensitivity and specificity were 86.15% and 79.59% for MCP-1, followed by 65.4 and 75.5 for procalcitonin, and then finally lower sensitivity and specificity in cases of CRP 52.5 and 64.3 respectively, for CRP.
Our results showed that MCP-1 concentration was significantly elevated in ascitic fluid of SBP patients compared to non-SBP with a strong positive correlation with PMNL. Salama et al. [25] reported similar data that ascitic fluid MCP-1 was higher in SBP than non-infected ascites and this is in agreement with the finding reported by El-Toukhy and Emam [26]. Also, Kim et al. found that ascitic fluid MCP-1 was higher in SBP patients and its level rapidly decreased after treatment [27] which supported our finding. In our study, ascitic fluid procalcitonin was significantly elevated in patients with SBP than non-SBP, with a positive correlation with PMNL, which is the gold standard for the diagnosis of SBP. These results agree with the result from previous studies that reported high ascitic fluid procalcitonin in SBP patients [24, 28]. Also, Yang et al. [29] analyzed 339 patients with SBP from seven previous publications reporting serum procalcitonin levels is a relatively sensitive and specific test for the diagnosis of SBP. Only one study reported no significant differences in procalcitonin levels between patients with SBP and those with sterile ascites [30]. However, only 10 patients with SBP were included in this study and consequently, the relevance of its findings is limited. Also, our data showed that CRP was significantly higher in SBP patients compared to those without SBP, with a weak positive correlation with PMNL. This result was in agreement with many studies that reported that CRP was higher in SBP patients [31, 32]. Although the basic level of CRP in cirrhotic patients is high, but when infection occurs, there is more deterioration in liver dysfunction and the increase in CRP is lower [14].
Our results revealed that the sensitivity and specificity (86.15% and 79.59% respectively) of MCP-1 were higher than Procalcitonin and CRP in the diagnosis of SBP. Also, El-Toukhy and Emam [26] mentioned that the sensitivity and specificity of MCP-1 were 86.7% and 95.4% respectively at a cutoff of 122.5ng/ml. A similar result was obtained by Elshayeb et al. [33]. Lin et al. mentioned in a meta-analysis that both procalcitonin and CRP are far from being a “gold standard” test, but when compared with patients with normal liver function, both procalcitonin and CRP have acceptable accuracy for diagnosing infection in patients with cirrhotic liver [34]. However, the usefulness of ascitic fluid procalcitonin levels for differentiating between SBP and those without SBP is still uncertain because of the small number of studies [22]. In this study serum creatinine was elevated in patients with SBP and this agree with Jun et al. [35], who mentioned that renal dysfunction is associated with SBP and an independent predictor of mortality in SBP. It is worth noting mentioning that Elsadek, et al. [46] found that the PEC index is an accurate non-invasive diagnostic tool for SBP in cirrhotic patients.
CONCLUSION
From the previous data we, concluded that Serum CRP, ascitic procalcitonin and MCP-1 were significantly higher in SBP patients compared to sterile ascites. Ascitic MCP-1 has a better diagnostic value with higher sensitivity and specificity in diagnosis SBP compared to CRP and procalcitonin which has higher diagnostic accuracy than CRP. Our study was limited by a small number of patients from a single center, also the lack of follow-up and relation to morbidity and mortality of SBP patients. So, further studies with a large number from different centers will be necessary to evaluate the usefulness of these markers in diagnosis, follow-up and relation to morbidity and mortality of SBP patients.
LIST OF ABBREVIATIONS
| CRP | = C-Reactive Protein |
| MCP-1 | = Monocyte Chemtactic Protein |
| SBP | = Spontaneous Bacterial Pertonitis |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the institutional ethical committee of Tanta University, Faculty of Medicine with approval number 30271\04\015.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All human procedures followed were in accordance with the guidelines of Helsinki Declaration of 1975.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants of this study.
STANDARDS OF REPORTING
STROBE guidelines were followed in this study.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


