AIMS65 and PALBI Scores as Predictors of Six Months’ Mortality in Cirrhotic Patients with Acute Variceal Bleeding
Abstract
Background & Aims:
Bleeding gastroesophageal varices are a cause of high mortality among cirrhotic patients. We aimed to investigate late mortality predictors and prognostic models using easily verified factors at admission in cirrhotic patients with acute variceal bleeding (AVB).
Methods:
Between January 2020 and June 2020, 142 patients with AVB from Tanta university hospital were included. Investigating multiple prognostic models was done using multiple logistic regression after identifying significant predictors of 6 months' mortality. Mortality prediction accuracy was assessed with area under the receiver operating characteristic (AUROC) curve.
Results:
The 6 months’ overall mortality rate was 31% (44 patients had died). AIMS56, Child-Turcotte-Pugh (CTP) grade C and MELD scores were significantly higher among non survivors (p<0.001) while Platelet-albumin-bilirubin (PALBI) was significantly more negative among survivors (P=0.001). Hepatocellular carcinoma was not significantly related to the mortality (p =0.364). Univariate analysis showed that high CTP, MELD, AIMS65 and PALBI scores were predictors of mortality and associated with decreased survival with high sensitivity and low specificity; while multivariate analysis showed that only AIMS56 was independently associated with mortality (p 0.004).
Conclusion:
CTP, MELD, AIMS65 and PALBI scores are simple, bed side risk scores that can be used for the prediction of 6 months’ mortality after AVB in cirrhotic patients with high sensitivities and lower specificities.
1. INTRODUCTION
One of the complications of acute decompensation of cirrhosis that occurs due to increased portal hypertension and hepatic dysfunction is acute variceal bleeding (AVB) [1], which is considered a leading cause of death in patients with cirrhosis. Despite multiple procedures are available to control bleeding, still AVB is associated with high morbidity and mortality in affected patients as it is associated with a 6-weeks mortality rate of 10 to 20% [2, 3].
This raised the need for the development of sensitive and specific risk prediction models for high-risk patients with AVB in order to decrease this mortality rate. A lot of prognostic models were developed such as; Rockall, Glasgow-Blatchford, Child– Turcotte-Pugh (CTP), Model for end-stage liver disease (MELD) and chronic liver failure-sequential organ failure assessment (CLIF-SOFA) scores, with The CTP score and MELD score being the best-known prognostic models [4].
However, these two models have some drawbacks for example ascites and encephalopathy in CTP scores are subjected to inter-observer variability [4] While MELD score has the advantage of not being subjected to inter-observer variability like in the case with the CTP score [5]. However, it has several limitations as it can be affected by causes other than liver diseases [6, 7]. This raised the need for the development of new scores that lack those limitations. In addition, it has poorer discriminatory power in the lower ranges of the score [8-35]. Two scores had been proposed as simple and accurate for the prediction of portal hypertension severity and in-hospital mortality [9, 10], Platelet-albumin-bilirubin (PALBI) score and AIMS65 score [10], as they are considered an easily calculated bedside risk scores which depend on data routinely available at initial evaluation. But, PALBI score was utilized mainly for the prediction of re-bleeding and early mortality [11].
As the aim of most of the previous studies was to assess the risk of bleeding and early mortality, the main aim of the present study was to assess different simple easily applied prognostic models for detecting the risk of late mortality in patients with AVB.
2. MATERIALS AND METHODS
Study design: This study was a prospective study carried out on cirrhotic patients who presented with acute upper GIT bleeding admitted to Tropical Medicine Department, Tanta University Hospital from January 2020 to June 2020.
Patients with cirrhosis and acute bleeding from both esophageal and gastric varices or both confirmed by esophagogastroduodenoscopy (EGD) were considered eligible for this study. While patients under 18 years of age, those who did not undergo EGD, missed follow-up within 6 months from initial endoscopic examination, patients with previously manipulated varices, those on Beta blockers, or patients with upper GI bleeding due to causes other than ruptured varices (e.g, peptic ulcers and erosions, esophagitis, malignant masses and vascular ectasia) were excluded.
All patients participating in the study provided a signed informed consent. The study protocol followed the guidelines of the Declaration of Helsinki 1975 and was approved by the Tanta University Research Ethics Committee (approval code: 34326/12/20).
Methods: complete history and clinical assessment including; assessment of complications of cirrhosis, associated comorbidities, baseline demographic characteristics, and laboratory tests including measures of hemoglobin, white blood cell count, platelet count, serum blood urea nitrogen, creatinine, prothrombin time or international normalized ratio (INR), total bilirubin and albumin were recorded. Bleeding focus and endoscopic findings were described by the endoscopists who performed the EGD (E.M and M.L).
Medical management included patients using vasoactive drugs, antibiotics, blood transfusion, combined with endoscopic variceal ligation (EVL) (as it is the standard of care in treating AVB) [12] or injection sclerotherapy according to the site of bleeding was done as needed. A Sengstaken–Blakemore tube was placed when necessary. Transfusion requirements were defined as a number of packed red blood cells (pRBC) products transfused on the day of bleeding or transfused continuously during the following hospital stay.
All of the included patients had ultrasound on the abdomen and pelvis for the diagnosis of cirrhosis and the detection of hepatocellular carcinoma and other complications of cirrhosis. Computed tomography was done on the patients if needed.
The recorded baseline data was used to calculate various prognostic scores including;
The CTP score was calculated numerically as previously described using bilirubin, albumin, international normalized ratio (INR), and presence and grade of ascites and encephalopathy. Then patients were classified according to CTP score into class A if the score was 5-6, B if the score was 7-9, and C if the score was 10 or higher [13].
The MELD score was calculated as: 0.957 × loge (creatinine mg/dL) + 0.378 × log (bilirubin mg/dL) + 1.1 20 × loge (INR) + 0.643 [14].
PALBI score was calculated as: (2.02 × Log10 bilirubin) + [-0.37 × (Log10 bilirubin)2] + (-0.04 × albumin) + (-3.48 × Log10 platelets) + [1.01 (Log10 platelets)2] where bilirubin is in μmol/L and albumin in g/L, and platelet count in 1000/μL. PALBI was categorized as: PALBI 1 (score ≤ 2.53), PALBI 2 (score > 2.53 and ≤ 2.09), and PALBI 3 (score > 2.09) [9].
AIMS65: Age > 65 years (1 point), SBP ≤ 90 mmHg (1 point), altered level of consciousness (1 point), serum albumin < 30 g/L (1 point), INR > 1.5 (1 point) [10].
Then, patients were followed up for 6 months or until death and outcome data were recorded, including hospital stay, rebleeding, readmission, infection (in the first 5 days after hemorrhage due to either; spontaneous bacterial peritonitis, pneumonia, urinary tract infection (UTI), bacteremia, or other infection) that was diagnosed using clinical, radiological, or bacteriologic data.
2.1. Statistical Analysis
Data were analyzed by the Statistical Package for Social Sciences (SPSS) V. 23 (SPSS Inc. Released 2015. IBM SPSS statistics for windows, version 23.0, Armnok, NY: IBM Corp.). Data were expressed as Number (No), percentage (%) mean (x̅) and standard deviation (SD). A logistic regression model was used to assess the predictive factors of 6-month mortality. This model was considered a suitable alternative to the Cox model because the follow-up time was relatively short. Variables showing P-values <0.05 after univariate analysis and those that were considered clinically relevant were included in a multivariate logistic regression model to identify independent factors associated with 6-month mortality. For significant variables, coefficients and odds ratios with 95% confidence intervals were reported. Receiver operator characteristic (ROC) with respective points of maximal accuracy for sensitivity and specificity were generated to determine biomarker performance. Two-sided P- value of < 0.05 was considered statistically significant.
3. RESULTS
In this prospective study, 290 patients who presented with acute GI bleeding were screened for the possibility of enrolment, only 142 patients wh met our inclusion criteria and completed 6 months follow-up or died were enrolled and data of those patients were analysed. Of them, 42 patients had hepatocellular carcinoma (HCC) (Fig. 1).
The mean age of the patients was 58.46 ± 9.73 years (cirrhotic patients had mean of 57.26 ± 9.94 years, HCC patients had a mean of 62.14 ± 8.1457.5 years). There were 99 males (69.7%) and 43 females (30.3%). Viral hepatitis was the leading cause of liver cirrhosis in all the patients.
Two weeks of follow up showed that 8 patients developed an ulcer in follow up endoscopy mainly in those patients who had band ligation.
Infection was documented after endoscopy in 24 patients (7.3%), including 3 spontaneous bacterial peritonitis, 9 pneumonia, 2 UTI, 6 bacteremia, and 4 other infections.
Fourteen patients (14) out of 142 died at 6 weeks (9.8%),while the overall mortality at 6 months was 44/142 patients (31.0%, 95% CI: 23.2%-38.7%). Neither gender nor smoking was associated with mortality (p=0.359, 0.720 respectively). (Table 1)
As regards scores, AIMS56 and MELD scores were significantly higher among non survivors (2.18 ± 1.18 and 16.93 ± 7.31 respectively) than survivors (0.94 ± 0.73 and 11.52 ± 4.33 respectively) with p<0.001 for each. PALBI score was significantly more negative among survivors (-4.11 ± 1.03) than non survivors (-4.56 ± 0.97) (p =0.001). CTP grade C was significantly higher among non survivors as 29 patients had CTP grade C versus 14 patients in the survivor group (p <0.001). The presence of HCC was not significantly related to mortality (p =0.364) (Table 1).

Endoscopic management was done for 142 hepatic patients (107 with liver cirrhosis and 35 with HCC) presented with acute variceal bleeding then follow up for 6 months revealed that total deaths were 44 patients (31%) (31patients with cirrhosis and 13with HCC).
+HCC: Hepatocellular carcinoma.
| Marker | Mortality an 6 months | P value | |
|---|---|---|---|
| Non survivors (n=44) No. (%) |
Survivors (n=98) No. (%) |
||
| Gender Male Female |
33 (75.0) 11 (25.0) |
66 (67.3) 32 (32.7) |
0.359 |
| Smokers | 2 (6.5) | 8 (10.5) | 0.720 |
| Child Pough score A B C |
4 (9.1) 11 (25.0) 29 (65.9)* |
51 (52.6)* 32 (33.0) 14 (14.4) |
<0.001 |
| Liver status Cirrhotic HCC |
31 (70.5) 13 (29.5) |
76 (77.6) 22 (22.4) |
0.364 |
| Mean ±SD | Mean ±SD | ||
| AIMS56 | 2.18 ± 1.18 | 0.94 ± 0.73 | <0.001 |
| PAlBI | -4.11 ± 1.03 | -4.56 ± 0.97 | 0.001 |
| MELD | 16.93 ± 7.31 | 11.52 ± 4.33 | <0.001 |
| Marker | AUC | Cutoff | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|---|
| AIMS 56 | 0.798 | 0.50 | 90.9 | 27.6 | 36 | 87 | 47 |
| PALBI | 0.669 | -4.80 | 75.0 | 49.0 | 40 | 81 | 57 |
| Child Pough score | 0.808 | >A | 90.9 | 52.6 | 47 | 93 | 65 |
| MELD | 0.739 | 9.5 | 88.4 | 42.4 | 44 | 88 | 58 |
For predicting mortality, ROC curve analysis was performed, at cut off value 0.5 or more AIMS56 had 90.9% sensitivity, 27.6% specificity, 36% PPV, 87% NPV and 47% overall accuracy. Meanwhile, PALBI had 75.0% sensitivity, 49.0% specificity, 40% PPV, 81% NPV and 57% overall accuracy at cutoff point of -4.80 or more. CTP grade more than A, had AUC of 0.808 with 90.9% sensitivity, 52.6% specificity, 47.0% PPV, 93.0% NPV and 65% overall accuracy. MELD showed AUC of 0.739 with 88.4% sensitivity, 42.4% specificity, 44% PPV, 88% NPV and 58% overall accuracy (Table 2).
On performing univariate regression analysis, each of the four scores (Child Pough, MELD, AIMS56 and PALBI) was significantly associated with mortality (p <0.001, <0.001, <0.001 and 0.018 respectively). In comparison to grade A, CHILD Pough grade B had OR of 4.383 (95% CI: 1.285-24.946) and grade C had OR of 26.422 (95% CI: 7.946-87.780) to increase the probability of mortality. MELD score had OR of 1.174 (95% CI: 1.090-1.264), AIMS56 had OR of 3.980 (59% CI: 2.404-6.589) and PALBI had OR of 1.534 (95% CI: 1.076-21.59) of mortality. The multivariate regression model showed that only AIMS56 score was independently associated with mortality (p= 0.004) with OR of 2.422 (95% CI: 1.324-4.430) (Table 3 andFig. 2).
4. DISCUSSION
This prospective study was conducted to assess AIMS65 and PALBI scores among other scores as predictors of 6 months mortality in patients suffering from AVP.
The overall 6- months mortality was 31% as 44 patients died, 14 of them died during the first 6 weeks after the first attack of bleeding (9.1%). The 6 weeks low mortality rate was similar to other studies which recorded 12.9% to 15% mortality rates [15-17], while a higher mortality rate was previously reported in a study done in 1981 as 60% of their patients died after AVB [18]. Reduced AVB mortality in the context of cirrhotic portal hypertension is considered to be the result of advances in the management of varices and acute variceal bleeding.
| Score | Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | P value | OR | 95% CI | B | P value | OR | 95% CI | |||
| Lower | Upper | Lower | Upper | |||||||
| Child Pough grade* B C |
1.478 3.274 |
<0.001 0.018 <0.001 |
4.383 26.411 |
1.285 7.946 |
14.946 87.780 |
0.462 1.324 |
0.272 0.520 0.140 |
1.587 3.758 |
0.389 0.649 |
6.478 21.771 |
| MELD | 0.160 | <0.001 | 1.174 | 1.090 | 1.264 | -0.003 | 0.954 | 0.997 | 0.897 | 1.108 |
| AIMS56 | 1.381 | <0.001 | 3.980 | 2.404 | 6.589 | 0.885 | 0.004 | 2.422 | 1.324 | 4.430 |
| PALBI | 0.422 | 0.018 | 1.534 | 1.076 | 2.159 | 0.448 | 0.182 | 1.566 | 0.811 | 3.025 |
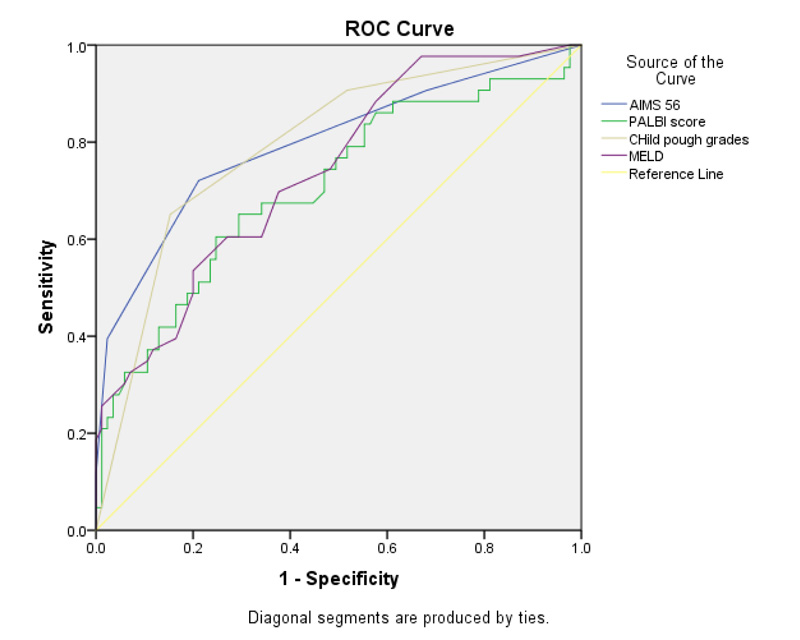
For predicting mortality ROC curve analysis was performed for the four studied scores. AIMS56 (blue) had AUC 0.798, PALBI (green) had AUC 0.669, CTP (brown) grade more than A had AUC 0.808 and MELD (purple) showed AUC of 0.739.
+AUC: area under curve.
Neither age nor gender showed any significant difference between the studied group. This was similar to a previous study [17], On contrary to our results, Charif et al. 2013 reported that advanced age was associated with early mortality this difference can be explained by different patients’ groups as 79% of their patients had esophageal varices without cirrhosis while all our patients were cirrhotic [19]. While Haukeland et al. found that the risk of death was positively associated was age and negatively with female sex this dissimilarity may be attributed to different etiological factors of cirrhosis [20].
HCC did not show significant differences between studied groups, on the contrary, previous studies found that concurrent HCC was associated with increased short-term mortality in patients with AVP. This difference may be due to advanced tumor status in their patients, in addition some of their patients had portal vein thrombosis leading to higher portal venous pressure [15-21].
Univariate regression analysis showed that all studied scores have a significant difference between studied groups. While AIMS56 score was the only independent predictor of mortality by multivariate analysis. ROC curve analysis showed that all scores had high sensitivity and low specificity in predicting 6 months’ mortality with AIMS56 and CTP scores, having the highest sensitivities.
In this study, the CTP score had a more predictive ability of mortality than MELD score. This finding was in disagreement with Sempere et al.,2009 and Hassanien et al., 2014 who concluded that the MELD score had a higher predictive ability than the CTP score and both had higher specificities and lower sensitivities than our study, this can be explained as both studies had different inclusion criteria as Sempere et al.’s study included patients with; previous renal impairment, tumor other than HCC, severe infection before admission, liver transplantation surgery in addition 4 of their patients were not scored in the first day of admission, while Hassanien et al. included only HCC patients with bleeding varices [16, 22], while Peng et al., 2014 concluded that CTP and MELD scores can equally predict mortality after upper gasterointestinal bleeding [23].
In agreement with the previous studies AIMS56 our findings were in agreement with the previous studies [10, 17, 18], which reported that the AIMS65 score was the simplest and most applicable scoring system for mortality prediction among cirrhotic patients suffering from AVB. In the same context, previous study reported that AIMS56 was an accurate, non-endoscopic risk score for the prediction of in-hospital mortality that can be applied early (within 12 hours of hospital admission) in patients with acute UGIB with a higher cutoff (≥2) [24].
In addition, a previous study had concluded that rebleeding and in-hospital mortality were accurately predicted by the AIMS65 score and that AIMS65 score of less than 1 can exclude rebleeding and they even concluded that the AIMS65 score was superior to other calculated scores in predicting mortality [25].
ALBI score was developed in 2015 by Johnson et al. in order to detect the survival of HCC patients after therapy [26], Then, PALBI score was developed by adding platelet count [27-29], and it was found to be a useful tool for the discrimination of patients with high risk varices obviating the need for unnecessary endoscopy in patients with HCC [11]. But the data about PALBI score as a predictor of mortality after AVB is still limited.
Elshaarawy et al.’s study showed that the PALBI score on admission was a good prognostic indicator for patients with acute variceal bleeding as regards early mortality and rebleeding with a higher performance than CTP and MELD scores [27].
To our knowledge, this is the first study to detect the role of PALBI score in six months’ mortality post AVB as the previous study by Elshaarawy et al. 2020 concentrated mainly on early rebleeding and early mortality [27]. One of the strengths of our study is a prospective study but the limitations of the study were the small number of patients and being a single-centered study. Larger number of multi-centric studies are needed.
CONCLUSION
AIMS56, MELD, PALBI and CTP scores may serve as good markers for exclusion of 6 months mortality risk in cirrhotic patients with acute variceal bleeding, With AIMS56 and CTP scores showing the highest sensitivities.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Tanta University Research Ethics Committee (approval code: 34326/12/20).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All human procedures followed were in accordance with the guidelines of the Helsinki Declaration of 1975.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants of this study.
STANDARDS OF REPORTING
STROBE guidelines were followed in the study.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
Dr. Sherief Abd-Elsalam is the Editorial Board Member of "The Open Biomarkers Journal".
ACKNOWLEDGEMENTS
Declared none.


