All published articles of this journal are available on ScienceDirect.
Applications of Different Biomarkers in the Diagnosis of Drug-induced Liver Injury
Abstract
Biomarkers perform a significant function in the process of drug development. Biomarkers have been utilized in the safety assessment of drugs in clinical practice and also for personalization of medicines. To recognize the relation among considerable biological processes as well as clinical outcomes, it is important to increase our potential of treatments for all ailments, in addition to our understanding of normal and healthy physiology. Since the 1980s, using biomarkers is essential for substitutional results in long term assessments of main maladies, for example, cancer, as well as illness related to the heart. Now a days, biomarkers are highly important for unifying discovery of the drug and day by day improvements. The importance of biomarkers is increasing gradually with the advancement of novel therapeutics for the treatment and prevention of a broad range of diseases in order to overcome hepatotoxicity. These biomarkers are extensively used for the identification of disease and the field of medical research. The use of biomarkers in clinical as well as basic research has been promoted rapidly by the different drug regulation authorities for better outcomes in the future.
1. INTRODUCTION
The term biomarker was introduced first in 1980 i.e. a biological marker, better known as “biomarker”, is a characteristic that is judiciously measured as well as considered as a sign of normal biological mechanism, pathogenic process or pharmacological responses to a curative intervention. Explanation of biomarker-defined by National Institutes of Health (NIH) in 1998 [1].
In the current scenario, biomarkers have performed a crucial role in the detection of the drug. Biomarkers can be used to understand the mechanism of the action of a drug, its effectiveness as well as indications of toxicity at primary stage of the development of pharmaceutical and in the diagnosis of patients likely to respond towards medication. Therefore, biomarkers have been utilized in the safety assessment of drugs in clinical practice and also in personalized medicines. However, some reliable biomarkers at the current time may predict who will be the positive responding group of patients, a group of non-responder patients who may be negative for the dose with the same intervention (Fig. 1). Hence, it is proved that there are huge studies and researches on biomarkers that are going on that some researchers have reported [2].
1.1. World Health Organization (WHO) Reports
- United Nations and the International Labor Organization coordinate the international programme for the chemical safety, which was organized by WHO and has given another definition of the biomarker, “any material, complex, or mechanism that can be measured in the body or its production in addition to anticipate or control the occurrence of ailment” [1].
- WHO has declared an appropriate explanation of biomarkers “The term “biomarker” is used in a broad sense to include almost any measurement reflecting an interaction between a biological system and an environmental agent, which may be chemical, physical or biological”. The considered feedback may be biochemical, functional as well as physiological at the molecular interaction or a cellular level, in the description of the efficacy of biomarkers in the assessment of environmental risk [3].
- Now a days, biomarkers are highly important for unifying discovery of the drug and day by day improvements. They are expected to be extensively used as an application in the examination of disease and surrogate restriction in medical research.

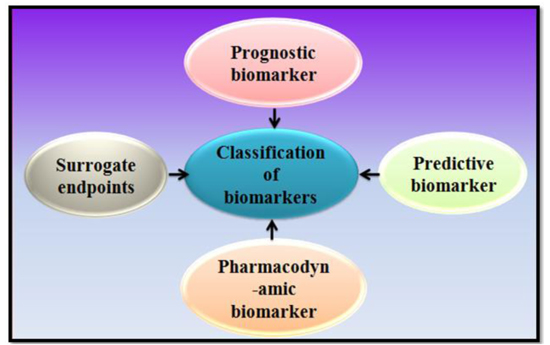
1.2. Classification
Classes of biomarkers are prognostic biomarkers, pharmacodynamic biomarkers, surrogate endpoints, and predictive biomarkers, etc. (Fig. 2) [4-10].
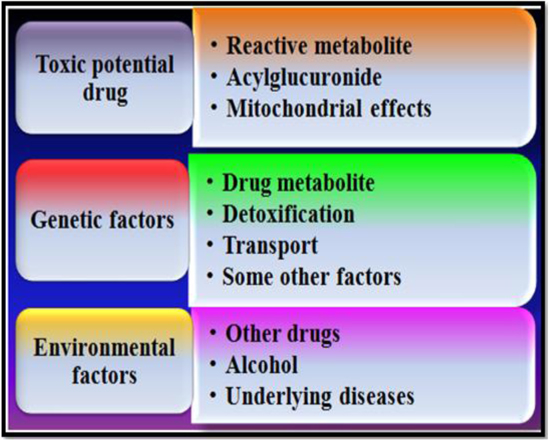
1.3. Risk Factors
Now a days, the risk of increasing hepatotoxicity consists of a complex reciprocal relationship among the chemical properties of the drug, sex, age, diseases as well as genetic factors (Fig. 3) [11, 12]. The most widely recorded risk factors belong to the use of the drug in addition to diseases. According to the current information for increasing liver toxicity induced by the drug in the patients with hepatitis B, hepatitis C illness and HIV which shows that there is an imbalance in the cytokines level of the infected person. The handling of the remedy for metabolism, detoxification, as well as transport along with those which influence the injury of the cell along with repair are controlled by genetic factors including genes [13]. The administration of toxicant considerably alters blood biochemical variables. Hepatic and renal Lipid Peroxidation (LPO) levels increase significantly. An extraordinary decline was noticed in the activities of Adenosine Triphosphatase (ATPase) and glucose-6-phosphatase (G-6-Pase) after induction of toxicity [14].
1.4. Drug-Induced Liver Injury (DILI)
The primary matter of medical practice is drug-induced hepatotoxicity. No doubt it is almost rare, in the US the leading cause of acute liver malfunction and transplantation of the liver is the drug persuaded injury of the liver [15]. The various marketed herbs, medication and dietary supplements are responsible for the liver injury. These market products have the potential to induce hepatic damage. In the preclinical investigations, it is found that around fifty percent of individual’s compounds exist in hepatic effects as well as in excess therapeutic dose [16]. The reason behind the failure of the drug in clinical trials is the DILI and usually is also responsible for the results in therapeutic activities as well as drug withdrawal [17, 18].
About 14 to 19 per 100,000 individuals of general populations are rated for drug-induced liver toxicity [19, 20]. While in an active and responsible system the frequency estimated is about thirty to thirty-three per ten thousand individuals [21]. The recorded incidence and harshness of DILI differ among drugs [20, 21]. It is suggested that the properties of drug achieved an immense role in the risk determination of DILI.
2. TYPES OF DRUG-INDUCED LIVER INJURY
- Intrinsic liver injury (Dose-dependent).
- Idiosyncratic liver injury (Non dose dependence).
DILI is generally divided into two parts i.e. intrinsic and idiosyncratic, means showed an effective performance of drug-induced toxicity vs. host factors in liver toxicity. However, the curve of dose-dependent influence towards the sensitization for toxicity at remedial quantities by the inflammatory stress which creates the two types of DILI are less distinct [22]. Indeed, approximately ten percent of acetaminophen is responsible for the cause of the cases of acute liver malfunction that occurred at approved dosage [23, 24]. Above and beyond, the dosage of the drug is a recognized determinant of idiosyncratic DILI [18, 25].
2.1. Drug Properties Linked to Risk of Drug-Induced Liver Injury
Drugs, according to the beneficial class vary on their hepatic accountability, physicochemical as well as toxico- logical properties that may influence the risk of DILI. Among properties of the drug, factors are also responsible for primary cell injury shown in Fig. (4) [26].

2.2. Biomarkers for the Diagnosis in DILI
Therapeutic mechanisms of necro-inflammation, fibrosis, apoptosis, steatosis, and oxidative stress are common to various types of liver damage or injury. The capability to explain these entities is beneficial for determining the mechanistic clue of adequacy, with the help of biomarkers for recommended treatment methods [27]. The stage of fibrosis at the index liver biopsy provides prognostic information about the following rate of fibrosis progression by the studies related to the natural history [28-30], and the expansion of liver-related conseq- uences [31, 32]. It is, as a result, no surprise that over the last decade much of the focus has been given to novel biomarkers based on the pathological incidence of fibrosis [33].
2.3. Graft-Derived Cell-Free DNA
In the process of transplantation of the liver, the recovery of the long term sufferer is still a big task. The advanced method for the analysis of the amount of DNA is very fast, cheap droplet digital PCR (ddPCR). Gc DNA is used for the early detection of transplant injury (“liquid biopsy”) as well as earlier implements additional efficient treatment intervention. It is beneficial at the time of the changes in immuno- suppression and to also guide in the minimization of immunosuppression. This new way for the detection of liver injury may also contribute to achieving more effective, lesser in the toxicity personalized immune suppression [34].
2.4. MicroRNA-155 and MicroRNA-196b
MicroRNAs (miRNAs) belongs to the small class, endogenous, noncoding RNAs that perform a serious emergency in the management of both processes pathological and physiological processes. According to some studies it is suggested that biomarkers may be involved in hepatitis C infection. The life cycle of HCV may affect miRNAs directly and indirectly in addition to the biological pathways critical for the progression of hepatitis C and HCV associated hepatic ailments. It is estimated that around three percent of the world populace is infected with HCV, and almost 350000 to 500000 citizens pass away every year from HCV associated hepatic ailments, for instance, cirrhosis as well as hepatocellular carcinoma. This shows that there is a high necessity of the progress to recognize markers that permit the monitor of Chronic Hepatitis C (CHC) succession in addition to recognizing patients who will not respond to treatment [35].
2.5. Alpha-Fetoprotein (AFP)
AFP is a type of the biomarker which is commonly used at the time of management of hepatocellular carcinoma (HCC). It is a type of glycoprotein which increased up to 70% in the blood of patients with HCC. The function of AFP in the diagnosis and surveillance of HCC has been extensively studied over the last five years [36].
2.6. Bilirubin
It is a type of a biomarker which had been investigated since the eighteenth century as in the prognosis as well as diagnosis of hepatic disorders, the estimation of the concentration of serum bilirubin is very important. It is a self-sufficient biomarker of mortality jeopardy hence proven according to some studies. An elevated level of total bilirubin is a sign of ailment, however, in the present time it ensures the association of a low level augmented in the menace of the disease [37].
2.7. Cortisol
Cortisol works as a cirrhosis biomarker and is known as the main glucocorticoid in humans. It is released from the adrenal cortex in the pattern of the dynamic and circadian manner, the hypothalamus-pituitary-adrenal axis monitors its secretion. It flows in the blood bound to carrier proteins, mostly corticosteroid binding globulin that is why it is also known as transcortin. Maintenance of the homeostasis is the function of Glucocorticoids [38].
2.8. CD133 and EpCAM
Two noteworthy factors which are used in the diagnosis of liver cancer is the repetition of tumor after the medical cure and resistance to chemoradiotherapy. Hypothesized, for initiation of tumor, recurrence, dissemination, and therapeutic resistance cancer stem cell is responsible. CD133 and EpCAM work as presumed markers of stem/progenitor cells in the liver. CD133 has indistinguishable usual physiological purpose although is recommended to be in intercellular communication. EpCAM has complex physiological functions like regulation of proliferation, cell-cell adhesion, migration, differentiation and survival of cells [39].
2.9. Hydroxyproline
Problems like viral hepatitis, bilharzia, metabolic disorders, and toxicity induced by the drug and chemicals are responsible for hepatic fibrosis, which is the most dangerous disorder of damaged tissues. The imbalance between the formation rate and collagen deposition are the two factors on which the proceeding of fibrosis depends. The activated Hepatic Satellite Cells (HSCs) are produced in the extracellular matrix by preserving the purity and function of liver cells [40].
2.10. Pentraxin 3
Pentraxins (PTX3) belongs to the family which having multifunctional pattern-identification proteins which are evolutionarily taking care of it. It is the prototype protein of the long pentraxin group, is a significant constituent of the humoral arm of innate immunity as well as opsonic activity, and facilitates pathogen recognition. In spite of the shielding utilities, PTX3 also participates in disease and fecundity of the females, the continuous increase in levels of PTX3 is reportedly correlated with the increased morbidity and severity of the disease in various clinical pathological conditions like as psoriasis, atherosclerosis, unstable angina pectoris and ischemic heart disorders [41].
3. LIVER BIOMARKERS FOR THE DEVELOPMENT OF NEW THERAPEUTICS
The importance of the liver biomarkers leads to the advancement of novel therapeutics for the treatment and prevention of a broad range of diseases to overcome hepatotoxicity. Just recently, liver fatty acid binding protein (L-FABP), as well as vitamin D-binding protein, has been diagnosed as biomarkers of liver toxicity and injury. After the detection of hepatic ailments, some natural therapies can be used to treat hepatic diseases, some drugs can also be produced at large scale for such diseases [42, 43].
3.1. Fibrinogen α-Chain
Liver fibrosis is the liver reaction to a misuse distinguished by an accumulation of extracellular matrix proteins. If the fundamental reason is not cured or eradicated, it may give progress to the ailment. Furthermore, it may also lead to various medical complications such as hepatocellular carcinoma or even to death. Thus, recognition, execution, as well as investigation of liver fibrosis are the major concerns in the detection as well as the healing of patients with chronic liver disease. In the present investigating years, new progression in mass spectrometry-based protein fractionation techniques, as well as proteomics technology, have boosted the identification of protein along with the quantification of numerous various samples of protein in addition to the ailments containing hepatic fibrosis [44].
4. SOME PROTEINS WHICH MIGHT BE USED AS LIVER BIOMARKERS
4.1. Soluble CD163
For the activation of kuffer cells, CD163 is a specific serum biomarker, which reflects the activation of kuffer cells at the time of inflammation as well as in oxidative stress in case of liver diseases and it belongs to ailment prognosis in addition to the treatment outcomes in several inflammatory circum- stances which affect the liver.
It shows the severity of liver diseases in the liver cirrhosis. CD163 in high levels that strongly belongs to the malady harshness along with the outcome is established in the conditions of high inflammation along with necrosis, for example, acute and chronic liver failure, as well as alcoholic hepatitis. It acquaintances with severity scores as well as portal hypertension, and it precisely forecasts sickness progression as well as survival (Fig. 5) [45].

4.2. YKL-40
It is found that the level of YKL-40 protein expression is augmented in patients with alcoholic liver disease as well as simultaneous chronic hepatitis C virus infectivity. It has also, been recommended that YKL-40 has also played the function in the development of cancer cells as well as survival, participates in the inflammatory progress in the region of the tumor as well as in angiogenesis. Serum YKL-40 was considerably related to the degree of hepatic fibrosis with the utmost levels in patients with mild to serious fibrosis. Patients with metastatic tumor with the poorest diagnosis have the highest level of serum YKL-40 (Fig. 6) [46].

4.3. Challenges in Using the Biomarkers
If biomarkers are utilized properly, the disease could be diagnosed at an early stage, and patients may undergo suitable treatment more rapidly, which may have enhanced outcomes at reduced costs. But regardless of the perspective in addition to the increasing potential, obstructions to complete use are present. Some challenges are identified in the use of biomarkers as the prospective necessity for a prognostic biomarker, confirmation from literature as well as preclinical, observational, in addition to untimely clinical research behind a strong biological justification for the biomarker as well as curative agent interface being prognostic of curative advantage, convene the entire appropriate diagnostic, pharmaceutical, in addition to regulatory representatives early in the development to ascertain performance necessities, the developmental path along with timeline, in addition to go–no go assessment points for the remedial agent as well as biomarker assay amalgamation. Well-defined progress goals along with milestones should be recognized for efficient go–no go decisions for curative agent as well as the biomarker assay [47].
4.4. Recent Developments
At present, no well-established blood-based biomarker available to diagnose some crucial diseases like Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), which troubles almost two million populations in the USA and in the world. The molecular deviations experienced in various investigations of ME/CFS blood cells propose the prospect to extend an investigative assay for samples of the blood. Taking benefit of current developments in micro/nanofabrication, direct electrical recognition of cellular as well as molecular possessions, in addition to microfluidics, developed an ultrasensitive along with economic nanoelectronic assay competent of continual examination of cellular as well as molecular events in real-time from a very minute volume of the sample approximate 50 µL) [48]. Interestingly, disulfide, dimethyl has been observed in recent times as a biomarker in the cases of melanoma [49]. Investigators have explored the panoply of prospective biomarkers, numerous of which would designate improper immune function as well as signs of autoimmunity, and some liver biomarkers are used for detecting the problems related to the liver (Fig. 7) [50, 51].
4.5. Requirements of Novel Biomarkers for Disease Diagnosis
In the field of medical science and research as well in clinical practice biomarkers have become very normal and common so it is now accepted almost without question in every clinical trial. In a variety of treatments and populations biomarkers predict relevant clinical outcomes so it has been used commonly and repeatedly, this use is entirely appropriate and justified. Biological markers that effectively evaluated and measured normal biological processes (heart rate, temperature, blood pressure), pharmacologic responses pathogenic processes (disease stage), or to therapeutic intervention as an indicator. From preclinical evaluations to drug recovery in each stage of clinical trials, the role of the biomarker has been increasing exponentially. To know the effect of therapeutic interventions, the progress of disease and toxicity induced by the drug, the interest in biomarkers as a biological indicator increase due to drugs fail during clinical trials because of high costs incurred. During the preclinical and clinical stages of drug development biomarkers also reduce attrition of drugs. Some investigations established the prospect of diagnosing as well as investigating the expansion of melanoma in mice by investigating the Volatile Organic Compounds (VOCs) in their urine. They confirmed that the VOCs present in the urine of the mice could offer the information about potential biomarkers of cancer in its untimely phases additionally, permitting for some stage of examining the progression of cancer. VOCs not used as just markers of the tumor but, as well, as markers of physiological alterations stimulated by an anti-cancer diet. These complexes could provide prospective biomarkers for differentiating between healthy, early, as well as late melanoma [52].
4.6. The Novelty of this Review
In this review, the emphasis has been given to the important role, which is performed by the biomarkers in the improvement of the process of drug development. To recognize relation among quantifiable biological processes as well as clinical resultant, it is important to increase our potential of healing for the entire ailments for our understanding of normal and healthy physiology.
CONCLUSION
If we entirely understand the usual physiology of a biological process, the pathophysiology of that process in the disease state, as well as possessions of an involvement pharmacological, piece of equipment, or otherwise on these progressions then biomarkers could only serve as true replacements for scientific relevant endpoints. Biomarkers utilized properly could make sure patients are identified in advance to the occurrence of the ailments, that they are coordinated to the suitable treatment more rapidly, as well as which has enhanced outcomes at reduced costs. The molecular deviations experienced in various investigations of ME/CFS blood cells propose the prospect to extend an investigative assay for samples of the blood. Taking benefit of current developments in micro/nanofabrication, direct electrical recognition of cellular as well as molecular possessions, in addition to microfluidics in the field of medical science and research as well in clinical practice biomarkers have become very normal and common so it is now accepted almost without question in every clinical trial. In a variety of treatments and populations biomarkers predict relevant clinical outcomes so it has been used commonly and repeatedly, this use is entirely appropriate and justified. Biological markers that effectively evaluated and measured normal biological processes (heart rate, temperature, blood pressure), pharmacologic responses pathogenic processes (disease stage), or to therapeutic intervention as an indicator.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTERESTS
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


