All published articles of this journal are available on ScienceDirect.
The Cytoglobin Expression Under Hypoxic Conditions in Covid-19 Cases
Abstract
Introduction:
Cytoglobin (Cygb) is an oxygen transporter marker that appears in hypoxic conditions. The clinical condition of Corona Virus Disease 2019 (COVID-19) cases, in general is that patients experience hypoxemia with low oxygen saturation. The Cygb gene is stimulated by the Hypoxia-Inducible Factor-1alpha (HIF-1α) transcription factor, which is stable in hypoxia.
Methods:
This study investigates Cygb expression in hypoxic COVID-19 cases. The design of this research is analytically observational. Parameters measured were Cygb mRNA and protein levels, correlation of HIF-1α and Cygb proteins in COVID-19 patients with Alpha, Beta, Delta, Omicron variants and negative control patients.
Results:
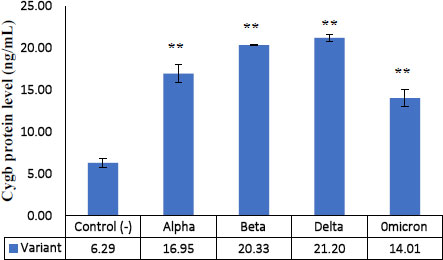
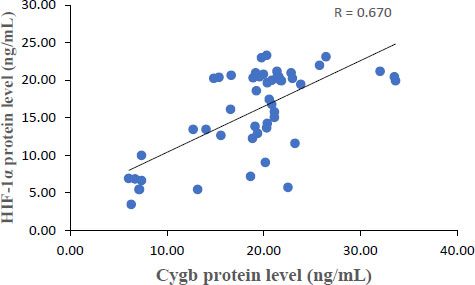
The results showed that each Cygb mRNA level decreased by 0.50, 0.92, 0.75 and 0.84 times that of the control. In contrast, Cygb protein levels (ng/mL) increased (16.95; 20.33; 21.20; 14.01 and 6.29 control). Strong negative correlation between mRNA and Cygb protein (R = -0.611). Strong positive correlation between HIF-1α and Cygb protein (R = 0.670).
Conclusion:
This study showed that Cygb mRNA expression decreased, further increasing Cygb protein; HIF-1α protein levels increased, further increasing Cygb protein. In COVID-19 patients (Alpha, Beta, Delta and Omicron variants), there is an increase in Cygb protein levels through stimulation of HIF-1α, which is stable under hypoxic conditions. The regulation of Cygb in this study has the potential to become the basis for handling cases of viral infections or other cases of hypoxia.
1. INTRODUCTION
Oxygen is a finite resource during the peak of the pandemic wave. All hospitals are running out of oxygen around the world. The failure of the oxygen system treatment causes an increase in the severity and even death of Corona Virus Disease 2019 (COVID-19) patients. The World Health Organization (WHO) estimates that an average oxygen flow rate in severe COVID-19 patients at a value of 10 L/min and saturation of <90% O2 is associated with increased morbidity and mortality. The availability of oxygen for COVID-19 patients during a pandemic is very low. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-COV-2) virus infection is a pneumonia virus resulting in hypoxemia and Acute Respiratory Distress Syndrome (ARDS). One effort of treatment is through the use of mechanical ventilation or oxygen therapy. The fulfillment of oxygen in COVID-19 is an effort to treat and overcome hypoxemia and protect against tissue hypoxia; the hope is to avoid damage to the body's vital organs [1].
In addition, it is also necessary to understand the cellular level of oxygen demand in hypoxic conditions. In the case of COVID-19, there is an increase in metabolism at the cellular, tissue and organ level. The results of this metabolism are used to increase defense against infection, focus on efforts to tolerate disease and survive with long-term metabolic processes [2]. This causes a high need for energy and O2 in COVID-19 patients [3]. In addition to SARS-COV-2 infection, there is an increase in viral activity and metabolism, which also requires high O2 for the invasion and replication of new virions [4]. With the high demand for energy and O2, while the oxygen supply is limited, the COVID-19 patient clinically shows low oxygen saturation (below 90% O2) and is said to be in a hypoxic condition [5].
The pathophysiology of hypoxemia in COVID-19 cases is caused by many factors, one of which is the stimulation of local inflammation, which results in interstitial pulmonary edema, the presence of microvascular thrombosis in the pulmonary blood vessels, which causes ventilation/perfusion mismatch [6]. Subsequently, from endotheliitis and vascular damage, there is impaired vasoregulation, loss of regulation of pulmonary perfusion, and increased hypercoagulability and thrombogenesis, leading to respiratory failure and ARDS [7]. All mutations of SARS-COV-2 produce various COVID-19 phenotypes, which can attack cells of the central nervous system and cause inflammation, disrupted interoceptive responses, new intrapulmonary additions, disrupted vascularity, and stimulated the formation of transcription factors involved in the adaptive response to hypoxia [1].
At the cellular level, hypoxia can cause disruption of cytosolic and mitochondrial metabolic mechanisms, which consequently causes a decrease in glycolytic processes, disruption of the electron transport chain, respiration chain and oxidative phosphorylation. As a result, the depletion of cellular adenosine triphosphate (ATP) stores is required for the activity of cells or organ tissues [8]. Hypoxia can also induce cellular apoptosis through inflammation releasing Reactive Oxygen Species (ROS), releasing apoptogenic proteins such as cytochrome c, inducing direct DNA damage, inhibiting the repair process of protein synthesis, activating the necrosis/apoptosis signaling pathway through p53, and upregulating the formation of Hypoxia-Inducible Factor-1 (HIF-1) transcription factor [1].
Cells experiencing hypoxia will induce stabilization of the protein Hypoxia-Inducible Factor 1-Alpha (HIF-1α) in the cytosol, which then combines with Hypoxia-Inducible Factor 1-Beta (HIF-1β) in the nucleus to become HIF-1 [9]. HIF-1 will then act as a transcription factor and activate various genes needed for adaptation, such as hypoxia that occurs in the case of COVID-19. One of the efforts to overcome hypoxia is through Cytoglobin’s (Cygb's) role in supplying O2 to all. Cygb levels are known to increase in hypoxia; its synthesis is regulated by HIF-1α through the binding of the Cygb gene promoter to the Hypoxia Response Element (HRE). hypoxia is generally accompanied by increased production of ROS, which regulates HIF-1α activity [10]. hypoxia is always followed by oxidative stress, which also activates HIF-1α synthesis [11]
Cytoglobin is an oxygen transport protein found in various tissues. In contrast to other globin families which have a specific location, such as hemoglobin (Hb) in erythrocytes, myoglobin (Mb) in striated muscle, and neuroglobin in nervous tissue; Cytoglobin is an intracellular protein equipped with a hexa-coordinated heme-Fe atom to iron and exhibits a binding affinity for one O2 molecule. Cygb protects various tissues from hypoxic conditions to varying degrees. Until now, the physiological role of Cygb has not been fully understood. Besides having the function of supplying O2 to cells, it may act as an O2-consuming enzyme or as an O2 sensor [12].
The role of Cygb, besides supplying oxygen in hypoxic tissues, is also thought to be able to reduce oxidative stress because it directly counteracts the radicals produced in hypoxia (ROS scavenger). However, over-expression of Cygb occurs in excessive cell proliferation, such as in malignancy; there is downregulation of Cygb expression due to hypermethylation of the Cygb promoter, as occurs in malignancy of the esophagus (tylosis) and squamous cell carcinoma of the neck and head [13].
Since COVID-19 was first reported on December 2020 in Wuhan, China, it has spread throughout the world and caused more than 750.000.000 cases and more than 6 million deaths globally [14]. The situation of COVID-19 in Indonesia until February 28th, 2023, is as follows, obtained 6,736,046 confirmed cases, 6,571,692 (97.6%) recovered, and 160,914 (2.4%) died. In February 2023, there were still 154 cases of death due to COVID-19 [15]. COVID-19 is caused by infection of SARS-COV-2, which has numerous variants based on its genetic differences as the result of viral RNA mutations from the original strain (Wuhan strain) [16]. Variant alpha, beta, delta, and omicron have appeared as a variant-of concern during the pandemic. According to several studies, each variant showed different characteristics related to transmissibility, severity, and lethality. Alpha, Gamma, and Delta variants were reported to be more severe and lethal than the Omicron variant, whereas the Delta variant is more easily transmitted, the symptoms are more severe, and cause more deaths [17].
Many countries are experiencing the second or third wave of outbreaks of this viral disease, and until now, mutant virus variants have continued to appear. At the end of the current pandemic, vaccine efficacy is still under WHO monitoring. Reports show that the classic four-dose mRNA vaccine (BT162b2 or mRNA-1273) has low research results and is short-lived in preventing SARS-COV-2 infection, especially in the omicron variant. The future of this infection and disease is unknown; researchers are still continuing to search for drugs that are effective against COVID-19 [18]. The variants of SARS-COV-2 are of concern to WHO, considering the number of cases of hypoxic patients, the length of treatment and the number of deaths that have resulted in global health problems, namely mutations of the Alpha, Beta, Gamma, Delta and Omicron type [19]. The alpha, beta and delta variants of SARS-COV-2 appear during the natural immune response of patients infected with an infection that has not been vaccinated. On the other hand, this omicron variant appeared at a time when vaccine immunity was increasing in the world. The transmissibility of delta variants is due to increased viral loads, longer duration of transmission, and high reinfection rates, due to their ability to escape innate immunity. As a result, the delta continued to drive new waves of infections and remained dominant during the fourth wave in many countries [20]. The omicron variant is highly mutated and carries a very high risk of infection, mutating extensively, carries a high risk of spike infection and higher reinfection. The existence of this omicron variant shows that the COVID-19 vaccine is not fully effective against SARS-COV-2 infection [21]. In cases of hypoxia COVID-19, there are individuals who experience recovery, but some require long treatment and even death. So the role of Cygb is not completely clear in the case of COVID-19. Information on Cygb's role, this research was conducted to assess Cygb's role in hypoxia in COVID-19 cases.
2. MATERIALS AND METHODS
This research is an observational design. This research used nose-throat swab samples and RNA stored in the COVID-19 laboratory at the Faculty of Medicine, Islamic State University Syarif Hidayatullah Jakarta. This laboratory is one of the references for testing COVID-19 during the pandemic in Indonesia. The number of samples used was from 40 patients who were confirmed positive for COVID-19, consisting of 10 Alpha, 10 Beta, 10 Delta and 10 Omicron variants. The sample control used 10 COVID-19-negative subjects. The patient has provided written informed consent for the study and publication. This research was approved by the Ethics Committee of the Faculty of Medicine, Islamic State University Syarif Hidayatullah Jakarta (No. B 034/F12/ KEPK/TL.00/5/2022).
Both RNA isolation and ELISA tests were performed at the Biosafety Level 2 Laboratory (BSL-2). Swab and RNA isolation were obtained from nose and throat swabs of five each patient confirmed positive for COVID-19 (Alpha, Beta, Delta and Omicron variants) and controls using negative samples for COVID-19. This study measured relative Cygb mRNA levels (qRT-PCR technique), Cygb dan HIF–1α protein levels with Human ELISA Kit of SunRed (201-12-0423) procedure) and their correlation. And analyze the correlation of Cygb with hypoxia marker (HIF-1α). Each test in this study was carried out in duplicate. This research performed the Pearson correlation test between Cygb mRNA and protein, also between HIF-1α and Cygb proteins.
RNA isolation using the SPIN-X Viral RNA Extraction Kit SD Biosensor Biotechnology (L41MSPNENRO) method. The ratio of RNA purity was measured by nanodrop with A260/A280 values >1.7 and diluted at a concentration of 200 ng/uL. RT-PCR technique to detect mRNA relative to KAPA SYBR FAST one-step qRT-PCR universal Kapa Biosystems Kit (KK4650) using LightCycler 480 II (Roche) for cDNA synthesis and PCR amplification. Primer for Cygb: forward 5'-CAGTTCAAGCACATGGAGGA-3', reverse 5'-GTGGGAA GTCACTGGCAAAT-3' with 250 bp of product [22]. The housekeeping primer used was 18S: forwards 5'-AGAAACG GCTACCACATCCA-3', reverse 5'-CCCTCCAATGGATC CTCGTT-3', with product 258 bp [23]. Melting temperature (Tm) curve for all primers using a temperature of 58oC. It is measured using the Livak method. The statistical analysis of this study used the Kurskal Wallis test because there were more than 2 group variables, the distribution was not normal and not homogeneous.
3. RESULTS AND DISCUSSION
The average relative expression of mRNA in patients with COVID-19 variants Alpha, Beta, Delta and Omicron each decreased by 0.50; 0.92, 0.75 and 0.84 times the negative control (Fig. 1) were significantly different. This indicates the possibility of a decrease in the transcription process or an increase in translation at the protein synthesis stage. Fig. (2) shows an average increase in Cygb protein levels in COVID-19 patients with Alpha, Beta, Delta and Omicron variants. This study shows that there is indeed an increase in the translation process to form Cygb protein. The translation process increases and is not followed by a transcription process; it is suspected that there is overexpression of the Cygb protein in COVID-19 patients with Alpha, Beta, Delta and Omicron variants. This study also demonstrated a strong negative correlation between Cygb mRNA and protein (Fig. 3), whereby lower levels of Cygb mRNA were used to form high levels of Cygb protein. Fig. (4) shows a strong positive correlation between HIF-1α and Cygb protein, indicating a relationship that increases in HIF-1α protein levels increase Cygb protein.

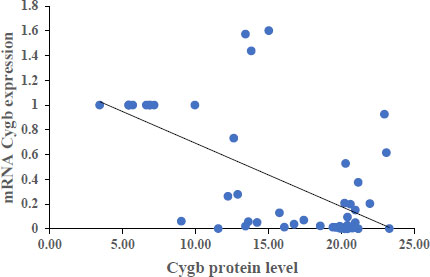
This study is not in line with studies on keloid tissue which identified hypoxia, where there was a strong positive correlation between HIF-1α protein and Cygb mRNA through the extraction of keloid tissue [24] and the growth of keloid fibroblast cells using the explant method [22]. This shows HIF-1α regulation at the transcriptional stage, in contrast to this study's HIF-1α regulation at the translation process.
Under conditions of low oxygen or hypoxia, the Cygb protein is synthesized to carry out the function of oxygen supply in all tissues. Cellular hypoxia marker through the presence of HIF-1α protein stabilization. HIF-1 is the first member of the HIF family to be expressed in all cells and tissues under hypoxic conditions. Likewise, Cygb as an oxygen carrier is also expressed in all vertebrate organs, especially vital organs such as the brain, liver, heart, lung, retina, intestine, and esophagus, as well as cells that have the potential to proliferate, such as hepatocytes, chondroblasts, and osteoblasts. Muscle, hepatocytes, epithelia and Hematopoietic Stem Cells (HSCs) [25]. The HIF-1 transcription factor is a heterodimer consisting of alpha and beta subunits. Under normoxic conditions, HIF-1α is degraded through hydroxylation by dioxygenase enzymes called prolyl-hydroxylases (PHD). This hydroxylated proline residue is recognized by the Von Hippel-Lindau (VHL) tumor suppressor, which is further polyubiquitinated and degraded through a proteasomal mechanism. In contrast, under hypoxic conditions, oxygen levels are insufficient for PHD enzymatic activation. As a result, HIF-1α is not degraded and is then translocated to the nucleus. Binding occurs to the HIF-1β subunit and the transcriptional coactivator p300. The HIF-1 transcription complex actively regulates the expression of all hypoxia adaptation genes (including Cygb). These genes are activated by binding to specific DNA called sequences Hypoxia Element Responses (HREs) [9]. This study also proves that Cygb has a strong correlation with the HIF-1α protein. The results of the correlation test in Fig. (4) show a strong relationship where an increase in HIF-1α protein levels will increase Cygb levels. The stable HIF-1α protein regulates hypoxia adaptation genes through binding to HREs.
The protein of Cygb is a gene regulated by HIF-1α, which physiologically tries to recover from hypoxic conditions by supplying O2. Adequate O2 supply causes normal cell metabolism and organ systems so COVID-19 patients can be cured. Cygb plays a role in maintaining the intracellular O2 supply under hypoxic conditions, but it can also act as an O2 reservoir or as a signal transducer in O2-sensing pathways. In neuroblastoma cells, overexpression of Cygb leads to cell overprotection and leads to cell death. Besides functioning related to O2 supply, Cygb also acts as a cytoprotective molecule because it has a heme prosthetic group under conditions of hypoxia and oxidative stress. Cygb scavenges ROS through detoxification mechanisms, and is involved in NO metabolism, protection against apoptotic mechanisms, and formation of lipid peroxides [26]. Cygb on superoxide catabolism has a strong superoxide dismutase (SOD) function. Cygb exhibits a high superoxide dismutation rate 10 times less than Cu, Zn-SOD. Cygb can quickly destroy superoxide in cells, providing protection against oxidant injury [27].
In another study, the expression of Cygb protein in cardiovascular aortic smooth muscle cells (aSMC) in rats under hypoxic conditions increased 40 times the expression of Mb and more than 200 times the expression of Hb. The main function of Cygb in muscle is to degrade Nitric Oxide (NO) to nitrite through cellular reducing systems and to maintain cardiovascular rhythms. The reducing Cygb expression in aSMC, there is a 70-75% reduction in NO metabolism in vascular smooth muscle. When NO reduction requires oxygen, Cygb plays a role in meeting the demand. Thus, inhibition of Cygb expression will lead to reduced oxygen availability resulting in a long process of NO decay, increasing vascular vascularity, and lowering blood pressure and vascular resistance. Cygb regulation in this regard is important in the treatment of cardiovascular disease [28].
Cytoglobin is a mechanism for protecting cells from apoptosis by stimulating an increase in the antioxidant glutathione, reducing total mitochondrial ROS, inhibiting caspase 9 activation, and then protecting mitochondria from oxidative stress fission. In addition, Cygb was reported to have peroxidase, superoxide dismutase, and nitric oxide dioxygenase activities. Cygb also suppresses etoposide-induced apoptosis in murine myoblasts, and doxorubicin-induced osteosarcoma cells degrade stimulated p53 [29]. In cultured HuMScs, cells can exert neuroprotective and anti-apoptotic effects via the mitogen-activated protein kinase (MAPK) signaling pathway p38 [30].
Cygb overexpression may function as a tumor suppressor. In cancer cell lines, Cygb can reduce cell migration, invasion, and growth. Cygb is unable to perform its function as a tumor suppressor in proliferating lung adenocarcinoma cell lines (H358). Thus, Cygb under normoxic conditions will still promote potential tumorigenic or carcinogenesis lung but depends on the cell type and microenvironmental conditions [31]. In hypoxia, Cygb overexpression decreases cell viability, augments migration, and suppresses cell proliferation under conditions of Cygb overexpression in COVID-19, it is suspected that SARS-COV-2 in the host has increased, which also utilizes O2 for the survival of the virus. Prolonged hypoxic conditions cause COVID-19 patient cells to no longer respond to the regulation of HIF-1α, resulting in a decrease in the transcription process. Prolonged hypoxia and overexpression of Cygb are thought to cause long hospital stays, organ failure and death in COVID-19 patients.
This study shows that Hypoxia and the transcription factor HIF-1α regulate Cygb expression in COVID-19 cases with Alpha, Beta, Delta, and Omicron variants. Further research is needed to elucidate the role of Cygb in viral infections, possible clinical implications in treatment and prevent the severity or mortality that occurs.
CONCLUSION
This study shows that Cygb mRNA expression is low and Cygb protein levels are high, indicating an increase in Cygb protein expression under hypoxic conditions in COVID-19 patients with Alpha, Beta, Delta, and Omicron variants. Potential Cygb protein biomarker for oxygen supply therapy in hypoxic conditions.
The limitation of this study is that the percentage of COVID-19 patients in this study could not be traced to experiencing recovery, prolonged hospitalization, or death. Only through a global analysis of the literature on the impact of the Alpha, Beta, Delta and Omicron variants.
LIST OF ABBREVIATIONS
| (ATP) | = Adenosine triphosphate |
| (ROS) | = Reactive Oxygen Species |
| (HIF-1) | = Hypoxia-Inducible Factor-1 |
| (HIF-1β) | = Hypoxia-Inducible Factor 1-Beta |
| (Hb) | = Hemoglobin |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This research was approved by the Ethics Committee of the Faculty of Medicine, Islamic State University Syarif Hidayatullah Jakarta (No. B 034/F12/KEPK/TL.00/5/2022).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
The patient has provided written informed consent for the study and publication.
STANDARDS OF REPORTING
STROBE guidelines were followed.
FUNDING
This study was funded by Research Funding Assistance from BOPTN Islamic State University Syarif Hidayatullah Jakarta (Proxy of Budget Users Islamic State University Syarif Hidayatullah Jakarta No. UN.01/KPA/223/2022).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


