All published articles of this journal are available on ScienceDirect.
Validation of a Predictive Model of Pre-eclampsia from a Cohort Study in Pregnant Women
Abstract
Background & Aims:
To validate a predictive model of pre-eclampsia for classifying pregnant women into pre-eclamptic and healthy groups.
Materials and Methods:
A cohort study was carried out in a total of 132 pregnant women, including biochemical and clinical parameters for assessing the classification performed by a predictive model of pre-eclampsia from a 10-fold cross-validation method and the experts’ criteria.
Results:
A highly predictive value was obtained from the set of biochemical parameters included in the proposed model. Wilks’ Lambda, eigenvalues, canonical correlation, and distances between centroids of the groups point to the high classificatory power of the discriminant function. Risk indexes, computed from the centroids, provided a measure of different risk levels for this condition. The analysis of these indexes in a prospective study allowed assessing the effect of the parameters. The new ten models obtained from a 10-fold cross-validation achieved a 100% of correct classification (AUC:1.00; CI:0.00-1.00; p=0.00). The sensitivity and specificity of the model, obtained from ROC curves, showed the consistency of the model, even though there were only four clinical manifestations of the entity. Moreover, the risk indexes were assessed from the experts’ criteria in the cohort study, showing an AUC of 100% in the 3rd trimester of pregnancy.
Conclusions:
The model and proposed pre-eclampsia risk indexes could constitute an accurate diagnostic tool, employing markers of oxidative stress as a significant element in the prediction of this entity.
1. INTRODUCTION
In pregnancy, one of the main causes of maternal mortality is Pre-Eclampsia (PE) [1]. It is considered a gestational syndrome, which has received significant interest worldwide. Around 50,000 women die each year due to causes related to this condition.
PE is characterized by an abnormal immunologicalvascular maternal response to the implantation of the product of conception, vasoconstriction, and metabolic changes in of conception, vasoconstriction, and metabolic changes in Nitric Oxide (NO), lipids and prostaglandins. Different studies have identified their relationship with Oxidative Stress (OS) [2] and its involvement in the pathophysiology of PE from the increase of Reactive Oxygen Species (ROS) [3, 4].
So far, these biomarkers have not been studied in pregnant women in association with the state of pre-eclampsia, neither using the approach of multivariate statistics for its early detection. For decades, some authors have used statistical models for predictive purposes in medicine to evaluate the role of risk factors, such as uterine artery Doppler and angiogenic markers, and to predict PE in nulliparous women [5-7]. The present work evaluates a predictive model developed from biochemical parameters currently used in clinical laboratories and related to the pathophysiology of this condition to assess the risk of PE in pregnant women.
2. PATIENTS AND METHODS
The validation of the proposed predictive model of PE [8] was carried out in a cohort study from January to December, 2019 in the obstetric service of three hospitals of Santiago de Cuba, Cuba. The aim was to assess the evolution of the risk indexes obtained from the predictive model.
2.1. Selection of Pregnant Women
A total of 132 apparently normal pregnant women, with ages between 21-35 years old, were included in the study after obtaining written informed consent. The institutional ethics committees evaluated the ethical aspects of this study. The diagnosis of PE was based on the following criterion of The American School of Obstetrics and Gynaecologists (ACOG): systolic arterial pressure ≥ 140 mmHg and/or diastolic pressure ≥ 90 mmHg taken twice within 4-hour intervals, proteinuria ≥ 300 mg in 24 hours in patients whose fetus age is at least 20 weeks and persistent oedema after 12 hours of rest in bed. Exclusion criteria for the pre-eclamptic group included nephropathies, autoimmune diseases, thrombophilia, diabetes mellitus, sickle cell diseases, and obstetrics conditions (gemelar pregnancies, hydatiform mole, fetal erythroblastosis, and polyhydramnios).
Demographic and clinical data were collected from interviews and clinical records of the pregnant women. PE in mothers, grandmothers, and other female relatives was considered as relevant clinical antecedents. Age, ethnicity, body mass index, number of spontaneous abortions, weight gain, toxic habits, consumption of nutrients, and others diseases were also considered as variables. The presence of edema and proteinuria were qualitatively evaluated. Exaggerated weight increase was considered when it exceeded 0.75 kg/week [1].
2.2. Biochemical Analysis
A volume of 5 ml was taken and carried out to obtain blood serum. A portion of this serum was conserved at -86°C for retrospective determination of Reduced Glutathione (GSH), Catalase (CAT) with an index of antioxidant defences, and Malondialdehyde (MDA) with an index of lipids oxidative damage [9]. Selected biochemical parameters were alanine Aminotransferase (ALT), Total Proteins (PT), Total Bilirubin (BT), Uric Acid (AU), Lactate Dehydrogenase (LDH), cholesterol and Triacilglycerides (TAG). All metabolites were processed with the chemical analyser HITACHI R 902 (Roche, Japan) in the clinical laboratory facility of the general hospital “Dr. Juan Bruno Zayas Alfonso” of Santiago de Cuba.
The antioxidant state in blood was determined with the spectrophotometer UV-T60 (Kaia Business International S.A) by a colorimetric reaction of 5.5’ ditio-bis 2-nitrobenzoic acid (DTNB) 10-2 M, producing a compound that absorbs light at 412 nm as a measurement of the concentration of GSH [10]. CAT was indirectly measured after the consumption of hydrogen peroxide and absorption at 240 nm [11]. The oxidative damage to lipids was measured at 586 nm after the reaction of the chromogenic reagent N-metil-2-phenylindole with a molecule of MDA at 45 °C [12].
2.3. Statistical Analysis
Statistical analysis of variables was achieved using GNU PSPP 1.0.1 (GNU General Public License, Free Software Foundation) with a significance level p < 0.05. The proposed predictive model of PE was obtained in a previous study [8] using Fisher’s Linear Discriminant Analysis (FLDA). In this study, a Ten-fold cross-validation [13] was used to evaluate the performance of the model constructed from FLDA functions to demonstrate its classificatory power. The divided data were successively tested into 10 different training (90%) and test groups (10%) that provided 10 new models with no significant differences in parameters and coefficients among them. Risk indexes of PE were obtained from the model.
A team of 10 obstetric experts classified pregnant women in the cohort according to their criteria during the three trimesters. Classification of experts and models were contrasted and presented as ROC curves to assess the clinical performance of the classification. To compare expert and model performances, only high risk was considered as confirmation of PE-diagnosed woman, which means that the model and expert assigned the highest possible value in the classification to the woman confirmed with PE.
3. RESULTS
A total of 132 female subjects were included in this cohort study. Twenty-two of them abandoned the study for reasons, such as abortions, relocation of residential address, etc. The function8 was represented in decreasing order by the coefficients: GSH, MDA, TAG, PT y LDH as:
D = 1.702 – 0.706GSH + 0.454MDA + 0.389TAG – 0.093PT + 0.007LDH
The Wilks’ Lambda and Fisher statistics demonstrated the consistency of the model, with a signification of p < 0.001 (Table 1). The confusion matrix showed that 100% of the originally grouped cases were correctly classified, showing specificity and sensitivity of 100%. The high value of the canonical correlation (95.8%) also indicates adequate membership of pregnant women falling into one of the a priori established groups according to expert criteria. The high eigenvalue associated with the discriminant function (11.047) explains 100% of the variance in the dataset. The centroids resume the best classification value for healthy pregnant (-1.807) and pre-eclamptic pregnant (5.873) [8].
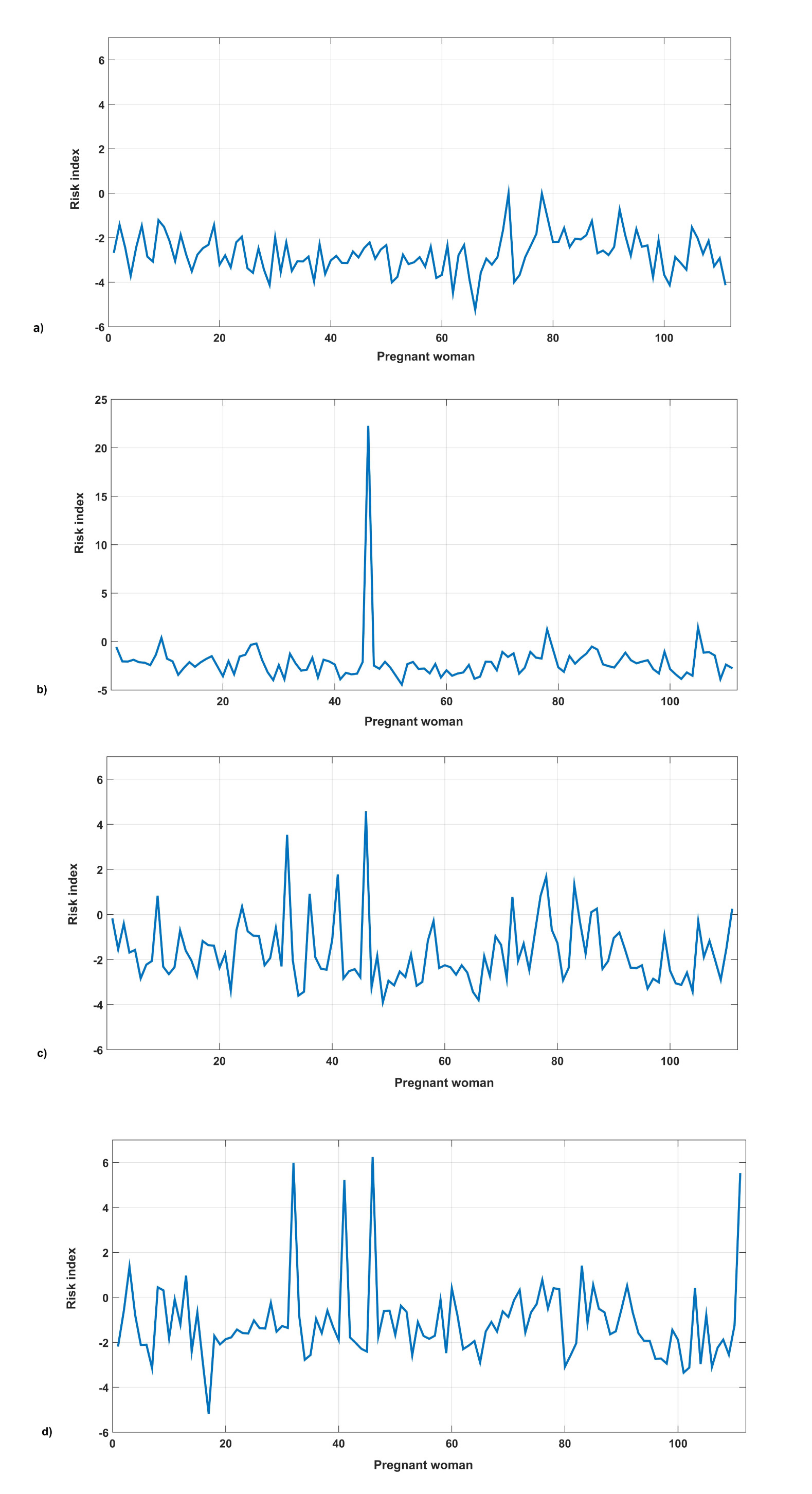
The risk indexes of PE were established, taking the values P1= 0.778 and P2 = 3.404 as cut points, scaled to the amplitude between both centroids. Three zones of risk were defined: minor, moderate, and major risk, as stated in Table 2. The time evolution of these indexes was evaluated in a cohort study (Fig. 1). Four pregnant women showed an elevated displacement with respect to the group average in the 3rd quarter, which placed them, according to their indexes, in the zone of severe risk for this entity. These women were later on diagnosed by specialists with aggravated PE.
| Step | Included variable* | Wilks’ Lambda | DF1 | DF2 | DF3 | Fisher | DF 1 | DF 2 | Sig. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MDA | 0,230 | 1 | 1 | 49,000 | 164.241 | 1 | 49,000 | p<0,001 |
| 2 | LDH | 0,156 | 2 | 1 | 49,000 | 129.707 | 2 | 48,000 | p<0,001 |
| 3 | PT | 0,107 | 3 | 1 | 49,000 | 130.560 | 3 | 47,000 | p<0,001 |
| 4 | GSH | 0,092 | 4 | 1 | 49,000 | 113.965 | 4 | 46,000 | p<0,001 |
| 5 | TAG | 0,083 | 5 | 1 | 49,000 | 99.422 | 5 | 45,000 | p<0,001 |
| Risk index | D range |
|---|---|
| Minor or Low | < 0,778 |
| Moderate | 0,778 – 3,404 |
| Major or High | >3,405 |
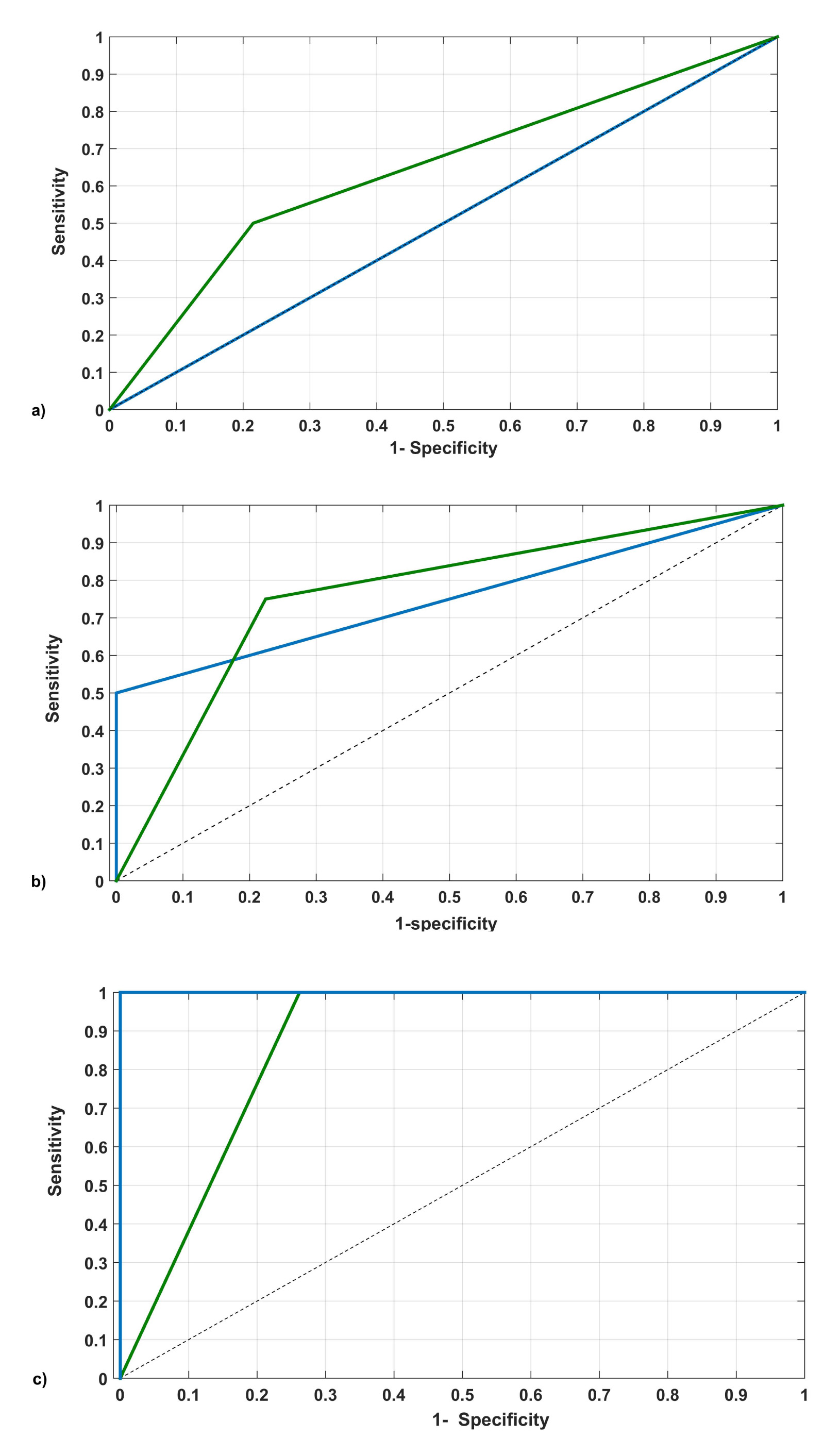
The new ten models obtained from a 10 -fold cross-validation achieved a 100% of correct classification (AUC:1.00, CI:0.00 – 1.00, p = 0.00).The classification of the experts contrasted to the model showed, through a “ROC curve” (Fig. 2), a good agreement in performance. As can be seen, from the II trimester, the original model agrees with the experts' criterion (AUC= 75% vs. 77%), reaching 100% in true positive by the III trimester. This performance shows the gradual improvement in sensitivity of the model (Table 3). Also, ROC curves show a gradual increase in sensitivity of both, the model and experts as the time of childbirth approaches (Fig. 2). However, experts exhibit a higher number of false positives and negatives than the model (Table 3).
4. DISCUSSION
The proposed model, obtained from a discriminant analysis, showed GSH, MDA, TAG, PT and LDH as the most significant classificatory biomarkers after a previous exploratory analysis using PCA. Good concordance with the scientific findings related to this entity was observed when evaluating the clinical value of each of them.
The presence of GSH in the function reflects its role in the physiology of pregnancy as an endogenous antioxidant and coincides with other studies that show its value in the diagnosis of severe PE [14]. This finding supplements theory of the depression of the antioxidant system to neutralize some reactive oxygen species, such as the radical O2 and H2O2, involved in the pathophysiology of PE. The inclusion of the MDA in the function has a valuable meaning in the usefulness of the model. Its role has been shown in discriminant analysis. Atiba [15] remarked the value of this metabolite as a marker of lipid peroxidation. However, it also includes TAG in the discriminant function as a precursor for complex lipids leading to a logical and statistical correlation with the oxidative process. It agrees with the previous results obtained by other researchers [16] related to the influence of the lipid peroxidation process (OS) in this entity.
Regarding PT, a strong association was found between low levels (hypoalbuminemia) and the appearance of PE in treated pregnant women (p < 0.001). Proteinuria in 24-hour urine shows renal deterioration caused by glomerular capillary endotheliosis, being the main cause of the decrease in PT in sera from affected women. The last component of the function is LDH, an enzyme that marks damage at the cellular level. Generalized vasospasm, vascular endothelial dysfunction, abnormal lipid metabolism, and insulin resistance can influence the liver and cause its increase, also resulting in a sign of microangiopathic hemolysis, and a marker of severity or HELLP (Hemolysis (H), Elevated Liver Enzymes (EL) and low platelets (LP) syndrome [17]. Ababio [18] showed that LDH exposure was associated with higher odds of the outcome in preeclampsia [OR (CI) =4.76 (1.26-18.72), p = 0.0068].
When observing Fig. (1), the D-value, obtained from the original model, shows a stable behavior (with values between -3 to 1, which places them in the normal state). However, pregnant women who have developed the disease show a significant difference after 30 weeks of gestation, which places them in an area of high risk. Therefore, this method allowed evaluating the time evolution of the indexes and the predictive value of the model since they were classified weeks before being diagnosed.
The evaluation of D-value for each pregnant woman in the validation group (Fig. 1) showed a 100% sensitivity in the classification. This is evidence of the consistency of the proposed predictive model and its practical value in the diagnostic of this entity. The sensitivity of the model in the original cohort (AUC: 1.00, CI 95%: 0.00 – 1.00) was coincident with the one obtained after the external validation. This is consistent with other studies [19]. Moreover, the indexes give information about the increasing model’s sensitivity with time. Fig. (1b) shows the increased index for the first pregnant woman, which is maintained for the successive evaluations (Fig. 1c, d). Around week 24, a newly pregnant woman is identified within the highest risk category (Fig. 1c). In week 30, two more pregnant women are added into the same category (Fig. 1d).
In general, a good agreement was observed when comparing the classifications of the model in the validation cohort with the expert criteria (Fig. 2). The model approaches the sensitivity of the experts by the third trimester, however, it appears to be more specific (Table 3). This probably reflects subjective factors involved in clinical diagnosis that is reconsidered during follow-up of pregnancy. From the model point of view, modification of biomarkers in the first weeks does not seem sensitive enough to differentiate normal pregnancy from oxidative unbalance in PE. It would depend on particular conditions present in the pregnant woman, however, in the second half of pregnancy, those markers could perfectly differentiate both states (normal pregnancy and PE), and it is reflected in the increased sensitivity of the model that allows detection of all cases of PE women in this study.
Finally, as it is known in pre-eclamptic women, endothelial damage intensifies over time, with alterations appearing in biomarkers undetectable in previous periods. The same happens with clinical aspects, which are nothing more than a manifestation of the organic damage that renders both methods (model and clinical diagnosis) to a good final correlation. The combined use would provide a more accurate classification during the follow-up of the pregnant women. Thus, the proposed model could provide a valuable aid for the obstetrician in cases of doubtful diagnosis.
Study limitations: the study had a small sample size of pre-eclamptic women. So additional studies at a larger scale are needed to validate our results.
| Methods | Cut Point | S (%) | E (%) | TP (%) | TN (%) | FP (%) | FN (%) | |
|---|---|---|---|---|---|---|---|---|
| Model | I T | 1,0 | 0 | 100,0 | 0 (0,0) | 107 (100) | 0 (0,0) | 4 (3,7) |
| II T | 0,5 | 50,0 | 100,0 | 2 (1,8) | 107 (100) | 0 (0,0) | 2 (1,8) | |
| III T | 0,5 | 100,0 | 100,0 | 4 (3,7) | 107 (100) | 0 (0,0) | 0 (0,0) | |
| Experts | I T | 0,5 | 50,0 | 75,6 | 2 (1,8) | 84 (75,6) | 23 (20,7) | 2 (1,8) |
| II T | 0,5 | 75,0 | 74,7 | 3 (2,7) | 83 (74,7) | 24 (21,6) | 1 (0,9) | |
| III T | 0,5 | 100,0 | 71,1 | 4 (3,7) | 79 (71,1) | 28 (25,2) | 0 (0,0) | |
CONCLUSION
The design of a predictive model of pre-eclampsia, including biomarkers associated with oxidative stress, made it possible to calculate predictive indexes for the severity of the disease. Under this assumption, it was possible to establish three risk categories for PE in pregnant women. The prospective application of the model in a cohort ensured a classification with 100% accuracy. The used validation methods showed the validity of the classification. The proposed pre-eclampsia risk indices could constitute an accurate diagnostic tool, employing oxidative stress as a significant element in the prediction of this obstetric entity.
AUTHORSHIP STATEMENT
All the authors participated sufficiently in the work and approved the final version of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethical Committee of the Obstetric Service of Mothers Hospitals of Santiago de Cuba, Cuba.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All patients participated on a voluntary basis and gave their informed consent.
AVAILABILITY OF DATA AND MATERIALS
The data sets used and/or analysed during the current study are available from the corresponding author [S.A.E] on reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest.
ACKNOWLEDGEMENTS
This work has been supported by the Belgian Development Cooperation through VLIR-UOS (Flemish Interuniversity Council-University Cooperation for Development) in the context of the Institutional University Cooperation programme with Universidad de Oriente.


