All published articles of this journal are available on ScienceDirect.
The Hidden Danger of Environmental Chemicals during the “Windows of Susceptibility” in a Woman’s Life – How can we use Intermediate Biomarkers to Improve Breast Cancer Prevention?
Abstract
Introduction:
It has been observed that many toxic environmental agents increase risk, accelerate development, or deteriorate the course of breast cancer (BC). In particular, endocrine-disrupting chemicals (EDC) are harmful to endocrine receptor actions and signaling in the breast tissue.
Usually, there is a long interval of time between the exposure to EDC and BC incidence, and this often represents a serious obstacle for effective BC prophylaxis. Notably, during certain periods of a woman’s life cycle, the BC risk is particularly elevated due to increased susceptibility to some EDC. These windows of susceptibility (WOS) include prenatal, puberty, pregnancy, and menopausal transition stages of a female’s life course.
Four WOS have been considered as the most vulnerable periods for BC since the mammary gland undergoes the main anatomical and physiological transformations at those intervals. This means that during specific WOS, the EDC from the environment can have the most dangerous impact on BC risk and possible BC development later in a woman’s life. However, most clinical BC studies related to toxic environmental exposures have not been connected to the specific WOS.
Therefore, the goal of this article is to briefly describe some important research results, focused on the links between EDC and BC, within four critical WOS. In addition, this mini-review outlines some useful biomarkers for further research and prophylaxis of BC and also for both the research community and the medical professionals.
Conclusion:
To bridge the gap in BC prevention, it is essential to recognize the links between EDC and BC within the critical WOS. Moreover, an integrative model of BC research, applying intermediate biomarkers, is necessary to determine the mechanisms of action of various EDC during critical periods in a woman’s lifespan. Hopefully, this will lead to progress in BC prevention.
1. INTRODUCTION
Despite enormous progress in the field of Breast Cancer (BC) research, primary and secondary prevention of BC still remains very challenging [1]. This is mostly due to the fact that several known risk factors for BC have only modest correlations with the BC prevalence [1]. This is opposite to some other common malignancies (e.g., cervical or lung cancer), which are strongly connected to certain predominant risk factors, such as human papillomavirus, in the case of cervical cancer, or tobacco smoking in the case of lung cancer [2, 3]. In addition, numerous BC risk factors are often inconvenient or difficult to address via public health interventions.
Furthermore, traditional models of BC causation were usually based on exploring just one BC risk factor or BC cause at a time. However, several such factors typically interact with each other, and thus, their combination can lead to BC [4]. Therefore, it is becoming obvious that the transdisciplinary research on the impact of the environmental exposures on BC etiology is of utmost importance [4].
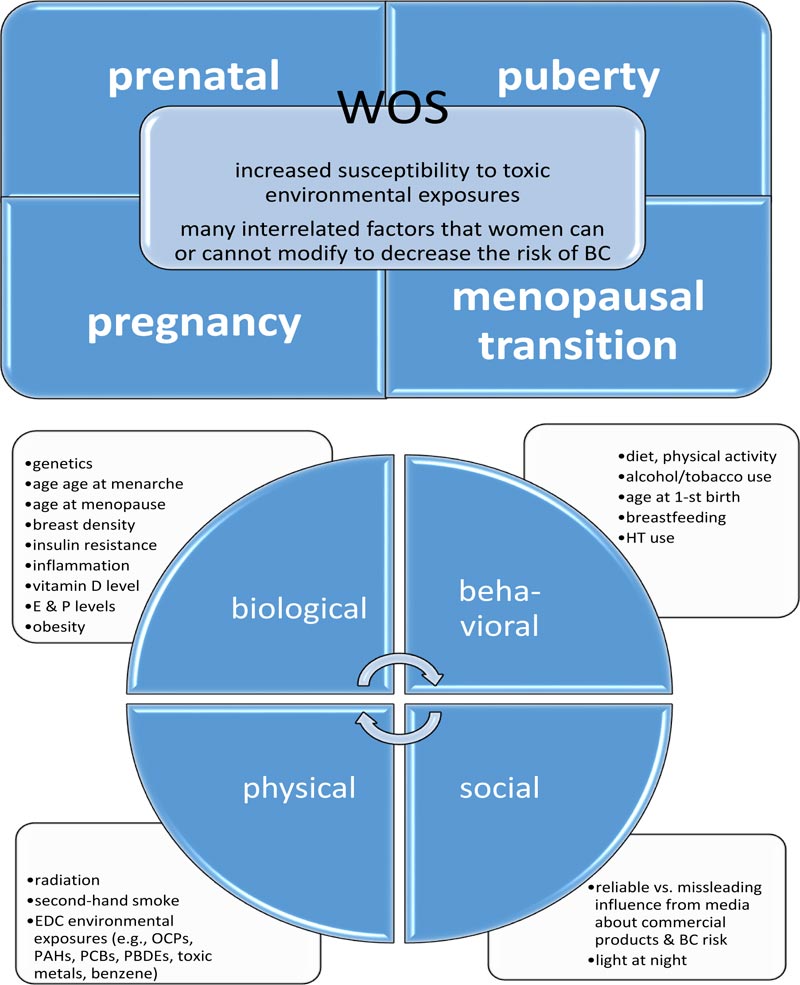
In fact, for many women, different risk factors for BC may still remain controversial or insufficient as targets for effective BC prevention [1]. At this point, longitudinal epidemiological studies can shed new light on the idea that toxic chemical exposures during some specific periods in a women’s life maybe crucial to the subsequent BC risk or development [5]. These so-called windows of susceptibility (WOS), involving the prenatal, pubertal, pregnancy, and menopausal transition periods, correspond with certain “mile-stones,” during which the mammary glands undergo anatomical and functional transformations [5]. In fact, when various toxic agents, derived from the environment (e.g., endocrine-disrupting chemicals (EDC)), and certain therapeutics (e.g., prescribed for the coexisting medical conditions or in the form of the hormone replacement therapy) act upon breast tissue during the particular WOS, they can influence BC risk, development, or outcome (Fig. 1) [5, 6].
In this paper, the evidence derived from epidemiologic studies focusing on the connections between environmental toxic chemical agents and BC within four critical WOS has been outlined. In addition, this mini-review provides some directions for ongoing and further research on intermediate biomarkers for BC. Moreover, the article describes an interdis- ciplinary research model, which combines input from the biological, behavioral, social, and physical domains that can serve as a useful tool for the exploration of environmental causes and risk factors, which can contribute to BC.
2. LOOKING FOR IMPORTANT NON-GENETIC RISK FACTORS AND CAUSES OF BC
Since “anything that is not genetic” can be considered as “environmental,” including many toxic agents, such as EDC, the relations between common chemical compounds and the risk or incidence of BC (or some intermediate outcomes related to BC, like age at onset of menarche) have revealed some important information [7]. EDC have been found in many industrial and agricultural chemical agents, as well as in numerous commercial and personal care products [7].
The most dangerous EDC include the following: organochlorines (e.g., Polychlorinated Biphenyls (PCBs) and dioxins), pesticides (e.g., Dichlorodiphenyltrichloroethane (DDT)), organohalogenated compounds (e.g., Polybrominated Diphenyl Ethers (PBDE)), per-and Poly-fluoroalkyl Substances (PFAS), Perfluorooctanoic Acid (PFOA), Phenols (e.g., Bisphenol A (BPA)), phthalates, parabens, Polycyclic Aroma- tic Hydrocarbons (PAH), benzene, ethylene oxide, certain metals (e.g., cadmium), and Dimethylbenz [a]Anthracene (DMBA) [6].
Furthermore, it should be noted that the excessive body mass, usually related to multiple reasons (e.g., elevated energy intake, poor quality of food, sedentary lifestyle, and decreased energy expenditure), and in particular, an increased body fat content, often share some BC risk factors with the environmental exposures [8]. An anthropometric parameter, such as the body mass index (BMI) (e.g., above 25, defined as overweight, and above 30, defined as obesity) has been associated with an increased risk of postmenopausal BC, a decreased risk of premenopausal BC, and an earlier age at menarche [9]. However, BMI is not the most accurate biomarker to evaluate body fat content. In fact, abdominal (central) fat is metabolically important with regard to insulin resistance and potential malignancy risk.
The paradoxical relationship of obesity (e.g., BMI above 30) in pre- versus post-menopausal women can be partially explained by the differential frequency of Estrogen Receptor-positive/Progestin Receptor positive (ER+/PR+) tumors, which can occur in these two age groups [10]. The ER+/PR+ tumors, which are more frequent among postmenopausal women, are more sensitive to Estrogen (E) that can be produced by the adipose tissue. In contrast, the ER−/PR− tumors are more common in the premenopausal population, and thus, such tumors can be due to some other risk factors (e.g., unrelated to the endocrine system). In addition, adipose tissue can serve as a reservoir for EDC, which is lipophilic and can be stored in the body for prolonged periods of time [11, 12].
3. ENDOCRINE AND CHEMICAL ENVIRONMENTAL INLUENCES ON BC RISK AND DEVELOPMENT, DURING THE SPECIFIC WINDOWS OF SUSCEPTIBILITY (WOS)
The four WOS are concisely presented in Table 1, including brief characteristics of physiological changes that typically occur in the breast tissue and the descriptions of potential harmful effects of EDC, which can take place during these critical periods [13, 14]. It should be noted that pregnancy may have a dual effect on BC risk. On the one hand, it has been known that a full-term pregnancy decreases the BC risk. On the other hand, however, some recent studies have reported that while the full-term pregnancy indeed lowers the risk for ER+ and luminal BC, pregnancy can also augment the risk of a very aggressive, basal-like BC subtype [15].
Based on preclinical studies, this can be related to the fact that the protective endocrine pathways may disturb by prolonged exposure to exogenous 17β-estradiol, which restores sensitivity to carcinogen-induced mammary tumors [16]. Since multiple complex interrelationships exist between age, ethnicity, parity, and body size (e.g., obesity), it is very difficult to clearly interpret and translate such data into the community audience (e.g., patients with BC, their families, and involved medical personnel). At this point, it should be highlighted that the tumor heterogeneity, especially in the case of the basal-like BC subtype, as well as the dynamic changes in the tumor microenvironment, promoting the development of basal-like BC during the post-partum period, plays important roles in this situation [15]. Furthermore, it has been known that both early pregnancy and multi-parity can decrease the risk of BC later in a woman’s life (e.g., post-menopause). In this context, the differentiation of the breast tissue caused by the physiological hormonal changes during pregnancy (e.g., increased progesterone level) has been considered to be protective against BC. Some complex mechanisms of the pregnancy impacting BC risk and development have been studied by investigating environmental chemicals (e.g., phenols), which were found in higher levels among pregnant females [17]. It is conceivable that such toxic agents may deteriorate the natural, protective pathways related to breast tissue rearrangement during pregnancy.
Some exemplary studies exploring correlations between the risk of BC and toxic chemical exposures from the envir- onment during particular WOS are summarized in Table 2. Notably, a prospective Child Health and Development Study (CHDS) is a landmark study connecting toxic environmental exposures during pregnancy and the future risk of BC [14]. In the CHDS, the DDT levels were measured in mothers soon after pregnancy, and then, these DDT levels were linked with the subsequent BC diagnoses established in their daughters (based on the documentation from medical records) [14].
Since BC is often hormonally stimulated, the physiological decline of female sex hormones, due to the decreased ovarian production, explains the reduction of the age-specific BC among post-menopausal women [18]. However, many BC cases are diagnosed after menopause, partially due to the augmented ER sensitivity at the time of menopausal transition. In addition, findings from the Women’s Health Initiative (WHI) study have revealed a higher incidence of BC, especially during the first year of hormonal therapy (HT) [19, 20].
In addition to chemical toxins, some metals, which activate the ERs, also known as metalloestrogens, represent as widely spread environmental contaminants that can accumulate in human tissues and organs [21]. With regard to BC, many studies have been focused on cadmium, which induces the proliferation of E-dependent BC cells, augments the transcription and expression of E-regulated genes, activates ERα, and increases cellular signaling via the ERK1/2 and AKT pathways [22, 23]. It is conceivable that the exposure to cadmium or other metalloestrogens, during any of the four WOS, may impact the BC risk via abnormal mitogenic signaling or inducing DNA synthesis in the BC cells [22, 24]. However, further studies investigating whether or not the exposures to certain metals or EDC during the particular WOS are related to BC risk are definitely merited, as well as exploring potential mechanisms of such correlations.
4. EXPLORING LONG LATENCY OF ENVIRONMENTAL CONTAMINANTS – IMPLICATIONS FOR USING NEW BIOMARKERS IN BC RESEARCH
The prolonged latency periods, from harmful exposures (during the prenatal, puberty, pregnancy, and menopausal transition WOS) to the BC occurrence, represent serious obstacles to introducing effective strategies for BC prevention. Unfortunately, many environmental toxins are lipophilic, and thus, they can be deposited in the adipose tissue reservoir (e.g., in the breast) for a long time [25]. It should be underscored that even though manufacturing of several dioxins, PCBs, and pesticides (e.g., DDT) was formally terminated in the 1970s, there might have been some exposures to such harmful agents due to their prolonged bioaccumulation in the animal’s adipose tissues, causing subsequent food contamination (Table 1) [25].
Processes of bioaccumulation of toxic agents and products of their metabolism in human tissues require further research, with the application of reliable biomarkers, which will reflect possible adverse changes in epithelial, adipose, and stromal tissue of the breast. In addition, it needs to be underscored that since the environmental chemicals can impact the onset and duration of the puberty or menopausal transition, research involving intermediate biomarkers (e.g., mammographic breast density (MBD)) should provide reliable information. Simply put, MBD presents the proportion of connective and glandular tissue to adipose tissue on a mammogram, and thus, it may serve as an intermediate outcome measure or possible marker or predictor of BC risk [26]. In fact, such parameters may even be more valuable than a girl’s age at menarche or a woman’s age at the beginning of menopause. Moreover, since many years could have elapsed from the toxic exposures to the establishing of BC diagnosis, the incorporation of MBD is crucial, particularly in prospective clinical studies.
One of the important diagnostic problems among women younger than 40 years is related to the fact that the information pertinent to the normal breast tissue appearance has been derived from mammography data of females over 40 years of age. Therefore, for a more accurate diagnostic work-up, in women younger than 40 years, different imaging methods should be applied to precisely evaluate the breast composition [27]. For instance, Magnetic Resonance Imaging (MRI) should be used for females 15–30 years old [28, 29] and Dual X-ray Absorptiometry (DXA) for girls 10 -16 years old [30]. Also, it should be noted that Optical Spectroscopy (OS), which displays a structure of the breast, reflecting its composition (e.g., the amount of water, hemoglobin, adipose, collagen, cellular and connective tissue density), can be very useful [31, 32]. In particular, collagen density can influence epithelial cell proliferation and enhance tumor invasion, while hemoglobin may affect angiogenesis [33, 34]. For instance, OS has been applied to non-invasively evaluate differences in adolescent breast tissue in different developmental Tanner stages during puberty [35]. In this way, MRI, DXA, and OS can serve as innovative, intermediate biomarkers to measure breast tissue changes during the developmental transformations in adole- scence or early adulthood. Moreover, these tests help overcome the inconvenience of long latency intervals of various EDC. They may also serve as valid tools for exploring EDC environmental influences during the breast developmental phases. In addition, some modern mammography techniques, such as digital breast tomosynthesis, as well as ultrasono- graphy, have been helpful in examining breast density in younger women without harmful exposure to radiation [27].
5. BRIDGING THE GAP BETWEEN THE NEEDS OF PATIENTS WITH BC IN THE COMMUNITY AND THE CURRENT BC ENVIROMENTAL RESEARCH
Unquestionably, community involvement can facilitate the implementation of BC research findings into daily practice. However, to determine the particular knowledge gaps and speed-up dissemination of the results of environmental BC studies, coordinated with particular WOS, it is essential to integrate efforts of the basic, clinical, public health, and communication research experts together with the medical providers and their patients with BC, or women at risk for BC, in the community. Such liaisons will be crucial since both the causes and the solutions for common toxic environmental exposures remain outside of the clinical settings.
At this point, an adequate community input may quickly detect problems, which pose particular concerns to several women at risk for BC. Moreover, a translation of new research data to the community members should enable a lot of patients with BC (or their female relatives at risk for it) to make informed decisions about their medical care, safe workplace, homestead or neighborhood, and healthy lifestyle. It should be highlighted that the personal involvement of many women with BC in the community partnerships has helped them learn about specific environmental hazards to their health and timely introduce the necessary modifications [36]. It should be highlighted that since many women are being diagnosed with BC above the age of 60 years, the effective prevention of EDC environmental exposures should be started in the early WOS. This requires new, proactive approaches to the patient’s education as well as the engagement in their own healthcare. Simultaneously, empowering physicians to spare no effort to prevent BC across generations in close cooperation with well-informed and motivated patients poses another big challenge [37].
6. PARADIGM II - AS A HELPFUL MODEL REFLECTING THE COMPLEXITY OF CAUSES AND RISK FACTORS FOR BC
In response to this challenge, the current model of the causes and risk factors for BC, Paradigm II, combines the complex interconnections between numerous components originating from four basic domains: biological, behavioral, social, and physical (relevant to the environment), which contribute to BC. In essence, Paradigm II reflects 96 potential dynamic interactions between these four domains [38]. Paradigm II was designed by a multidisciplinary team of experts of epidemiology, cancer biology, genetics, toxicology, public health, biostatistics, and computational modeling [38]. Although Paradigm II does not directly serve to diagnose and treat patients, it should be very useful for physicians and nurses as a modern educational tool for patients with BC, their family members, and caregivers. Its particular strength is related to the fact that it simultaneously focuses on the multiple interrelated causes and risk factors for BC that can be understandable and meaningful for many patients suffering from BC (e.g., family/genetic predispositions, exposure to toxic/carcinogenic chemicals at the workplace/house, and second-hand smoke) (Fig. 1) [5, 6, 38].
| WOS | Prenatal | Pubertal | Pregnancy | Menopausal Transition |
|---|---|---|---|---|
| The main changes in the breast tissue | Development of the mammary gland in the embryonic stage; epidermal cells & embryonic mesenchyme become breast buds [13] | Rapid growth of breast tissue; ductal tree & lobular structures formation; > ovarian E synthesis (> stimulation from hypothalamus & pituitary) leads to the onset of menarche [39] | Intense breast tissue/micro-environment changes in size & function before lactation; E, P, prolactin are crucial for branching & forming lobulo-alveolar structures [40] | Levels of E & P decline; however, mammary tissue can be more responsive to < levels of E & P via adaptation to the decreased ovarian production of these hormones [41] |
| Relations between ovarian E, P, EDC & neoplastic lesions in the breast tissue | Oscillations in maternal (E & P) hormones, > levels of growth factors, DNA damage, mutations in germ cells, genetic or epigenetic changes & acceleration of fetal growth or > birth-weight can augment BC risk later in life [42] | Excessive signaling through the ER can initiate mammary carcinogenesis; additional EDC influence the interaction of endogenous E with ER, contributing to carcinogenesis; environmental EDC may reprogram ER or normal stem cells [43, 44] | After a full-term pregnancy, breast cells are less sensitive to carcinogens in the long run; however, rare “pregnancy-associated BC” has usually worse outcome [45, 46] | Due to prolonged exposure to elevated E levels & EDC, late age at the onset of menopause can increase the BC risk [47] |
| Harmful effects on the endocrine system & BC development caused by common EDC | Organochlorines express endocrine activity; change breast tissue development, alter responsiveness to endogenous hormones & can promote neoplastic growth [14] | Pesticide (DDT) exposure during infancy & puberty is related to > BC risk later in life [48]; PFAS & PFOA enhance E effects of 17β-estradiol in T47D BC cells [49]; PFOA promotes the proliferation, migration/invasive potential of breast epithelial cells [50]; Phenols (BPA) [51], Organohalogenated compounds (PBDE) [52] & Phthalates [53] can delay puberty | PAH are stored in fat tissue of the breast; PAH reveal weak E properties & stimulate cell proliferation by activating the ERs [54]; higher levels of PAH-DNA adducts were reported in pts with BC [55]; elevated maternal exposure to DDT during pregnancy increased the daughters’ later BC risk [14] | PBDE modulate steroidogenesis (e.g., expression of aromatase) [56]; PBDE & BPA act as ERα ligands, contributing to BC development [57]; BPA can induce the proliferation of E-dependent breast cells & stimulate the growth of preexisting occult breast lesions to breast tumors [21] |
| Usual sources of the EDC | Organochlorines (PCBs & dioxins) - consumption of animal/fish fats, products from the contaminated areas (due to prolonged bioaccumulation of these lipophilic agents) [25]; PCBs - inhalation of the contaminated air (out-/indoor), dust from building materials, floor finishes, or caulk [58] | Pesticides (DDT) - consumption of animal/fish fats from the contaminated food [25]; PFAS - using commercial products characterized by non-stick, stain-resistant, waterproof features (e.g., common in industrial facilities, firefighting equipment, dietary products – fish or sea-food [59], food or water packaging [60]; Phthalates - using personal care products (e.g., cosmetics), plastics, and building materials [53] | PAH - consumption of grilled food (e.g., meats, fish) [61]; inhalation of cigarette smoke, industrial pollutions, or exhaust of vehicles [62, 63] | PBDE - using industrial products (e.g., flame retardants) [64]; Phenols (BPA) - using industrial agents that contain plastics, epoxy- resins, dental sealants, thermal paper, the lining of food containers, or beverage cans [65] |
| Abbreviations: BC, breast cancer; BPA, bisphenol A; CHDS, Child Health and Development Studies; DDT; dichlorodiphenyltrichloroethane; EDC, endocrine-disrupting chemicals; E, estrogen; ERs, estrogen receptors; P, progesterone; PAH; polycyclic aromatic hydrocarbons; PCBs; polychlorinated biphenyls; PBDE, polybrominated diphenyl ethers; PFAS, perfluoroalkylated substances; PFHxS, perfluorohexanesulfonate; PFOA, perfluorooctanoic acid; PFOSA, perflurooctane-sulfonamide; WOS, windows of susceptibility | ||||
Table 2.
|
WOS Toxic Agents Biomarkers |
Outcome Measures |
Study Population Average Age, Sample Size, Country, BC Risk Estimate |
Implications from the Research Study | First Author (Year) |
|---|---|---|---|---|
| Prenatal WOS Maternal DDT DDT |
Daughter’s BC | Mothers & adult daughters, 118 cases, 354 controls, California, U.S.; OR 3,7 | DDT is an endocrine disruptor & a marker of high BC risk | Cohn (2015) [14] |
| Pregnancy WOS Total suspended particulates at time of first birth |
Post-menopausal BC | Women 35–79 years old, 220 cases, 301 controls, Erie & Niagara Counties, Canada; OR 2,57 | Early life exposures influence BC risk & indicate the importance of toxic traffic emissions for BC risk | Nie (2007) [66] |
| Pregnancy WOS 16 serum PFAS 10PFCA,5PFSA,1PFOSA |
Pre-menopausal BC | 250 cases, 233 controls, Breast Cancer Danish National Birth Cohort, Denmark; PFOSA: RR 1.04 PFHxS: RR 0.66 | No convincing evidence for a causal link between PFAS exposures & premenopausal BC risk 10-15 years later was reported | Bonefeld-Jorgensen (2014) [67] |
| Pregnancy WOS Serum PCB during early postpartum |
BC before age 50 | Women in CHDS cohort, 112 cases, 112 matched controls, U.S.; PCB 167: OR 0.24 PCB 187: OR 0.35 PCB 203: OR 6.34 |
Postpartum PCB exposure may also represent pregnancy exposure; it can predict > risk for early BC | Cohn (2012) [68] |
| Puberty WOS p,p’-DDT metabolites in serum taken after giving birth (initial DDT exposure before age 14) | a. BC before age 50 b. BC diagnosis at age 50-54 |
Women in CHDS cohort, U.S.; a.129 cases & 129 matched controls; OR 5,4 b.153 cases & 432 controls; OR 1,88 |
a. Exposure to p,p'-DDT early in life may > BC risk; b. p,p'-DDT was associated with BC; the risk of BC depended on the timing of 1-st exposure & age at BC diagnosis, suggesting that the induction period starts early in life; DDT is an endocrine disruptor |
Cohn (2007; 2019) [69, 48] |
| Puberty WOS Urinary phenols |
Age at breast dev | Girls 6-8 years of age (followed for 7 years), 1239 girls, U.S.; Enterolactone: HR 0.79 Benzophenone-3: HR. 0.80 Triclosan: HR 1.17 2,5-dichlophenol: HR 1.37 |
Association of breast development with enterolactone, but not the other phenols, was mediated by body size; some phenols may be antiadipogens (benzophenone-3, enterolactone) or thyroid agonists (triclosan, 2,5-dichlorophenol); their high levels in girls should be explored | Wolf (2015) [51] |
| Puberty WOS Low & high MWP metabolites from urine |
Age of breast & pubic hair dev | Girls 6-8 years of age (followed for 7 years), 1239 girls, U.S.; Pubic hair dev age:HR 0.91 Breast dev age: HR 0.99 |
Phthalates as hormonally active pollutants can change the time of puberty | Wolf (2014) [53] |
| Puberty WOS Low & high MWP metabolites from urine |
Stage of breast/pubic hair dev | Girls 6-8 years of age (followed for 1 year), 1151 girls, U.S.; Pubic hair dev: PR 0.94 Breast dev: PR 1.03 | Weak hormonally active xenobiotic agents had minor associations with pubertal development | Wolf (2010) [70] |
| Puberty WOS PBDE, PCB, OCP |
Tanner stage 2+ vs 1+ breast dev | Girls 6-8 years of age (followed for 7 years), 645 girls, U.S.; PBDE: TR 1.05 PCB: TR 1.05 OCP: TR 1.10 |
Windham (2015) [52] |
Furthermore, the Paradigm II convincingly summarizes the updated knowledge about the relationships between various modifiable factors, which can be controlled or eliminated by individual women (e.g., tobacco smoking, alcohol consump- tion, and excessive intake of fatty, sweet, or highly processed food, lack of regular exercises, hormone replacement therapy, and use of toxic personal care products). In addition, some specific spheres of public health, which are related to social, economic, and industrial factors, which can have a profound adverse impact on women’s health, have been outlined. Hopefully, such a comprehensive and persuasive model will encourage both women with BC (or at risk for it) and medical teams in charge of their care to be actively involved in the preventive and therapeutic processes, utilizing intermediate biomarkers. Moreover, Paradigm II should inspire further research projects since it interactively presents the ever-changing relationships and often surprising manners by which the various causes and risk factors for BC can interact together and affect each other, depending on the changing circum- stances, during lifespans of individual women.
CONCLUSION
The biological alterations in breast tissue structure and endocrine signaling during the prenatal, pubertal, pregnancy, and menopausal transition WOS reflect the elevated BC risk at these particular periods. Therefore, measuring the effects of toxic environmental exposures during the four WOS is crucial to understand their impact on further BC risk and development. Notably, some epidemiologic studies have shown a connection between human exposure to toxic chemical agents, during critical WOS, via the application of intermediate biomarkers (e.g., MBD, especially in adolescent and young adult patients).
An integrative model of BC research is crucial to determine the influence and mechanisms of action of various EDC during critical periods in a woman’s lifespan. Hopefully, detecting the exact biological role of various EDC (originating from certain environmental exposures) at the specific WOS will contribute to measurable progress in BC prevention and therapeutic management of many women with BC or at risk for it.
For the most accurate exploration of several interconnected environmental causes of BC, an interdisciplinary BC research model, Paradigm II, combining input from the epidemiology, basic, and communication sciences, should be instrumental. Consequently, a close collaboration between researchers, medical practitioners, patients with BC, and the local community representatives is necessary. In this way, the newest research results in this area, applying intermediate biomarkers, would have a chance to be rapidly disseminated to the medical teams and the most vulnerable patients with BC, or women at risk for it, as well as their families or caregivers.
LIST OF ABBREVIATIONS
| BC | = breast cancer |
| EDC | = endocrine-disrupting chemicals |
| OCPs | = organochlorine pesticides |
| PAHs | = polycyclic aromatic hydrocarbons |
| PCBs | = polychlorinated biphenyls |
| PBDEs | = polybrominated diphenyl ethers |
| E | = estrogen |
| P | = progesterone |
| HT | = hormone therapy |
| WOS | = windows of susceptibility |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


