Plasma Fibrinogen as a Biomarker of Stable and Exacerbated Chronic Obstructive Pulmonary Disease
Abstract
Study Design:
An experimental, comparative, cross-sectional study
Place and Duration of Study:
Department of Physiology, Federal Post Graduate Medical Institute (FPGMI), Sheikh Zayed Medical Complex Lahore, Pakistan from August 2013 to 2014
Background:
Chronic Obstructive Pulmonary Disease (COPD) is a preventable and treatable disease, but is a partially reversible chronic inflammatory condition characterized by airway obstruction. COPD remains under-diagnosed and under-treated because the only available diagnostic method at present is testing lung functions by spirometry which is not helpful to determine the severity and clinical outcomes of the disease. Circulating biomarkers are under consideration for various diseases worldwide. Plasma fibrinogen is emerging as one of the most promising biomarkers of COPD in smokers.
Objective:
The objective of this study is to investigate if plasma fibrinogen can serve as a diagnostic biomarker of COPD in smokers, and if its further higher levels are seen in the exacerbated state of the disease in comparison to the stable disease.
Materials and Methods:
75 middle-aged to old-age smokers of either gender were selected. Lung functions of every participant were measured to determine Forced Expiratory Volume in the first second (FEV1), Forced Vital Capacity (FVC), and the ratio of FEV1/FVC by spirometry. On the basis of the results of the tests, subjects were divided into three groups; firstly, the control group of chronic smokers without COPD, secondly, smokers with COPD in a stable state, and thirdly, patients in an exacerbated state of COPD. Plasma fibrinogen was quantitatively estimated in every individual of all three groups by the Clauss method using the Hemostat Fibrinogen kit.
Results:
The average Plasma fibrinogen level was found to be 235.008 mg/dl in healthy smokers (control group), while an average of 440.12mg/dl was measured in patients with stable COPD. The difference in plasma fibrinogen levels was found to be significant, having a p-value of (0.000). In the third group with declined lung function predicting acute exacerbated COPD, fibrinogen was found to be > 453.2 mg/dl, which was significantly higher than in the stable disease group (p-value > 0.0017)
Conclusion:
Plasma fibrinogen level measurement is a reliable and accessible test in terms of a diagnostic marker of COPD, as compared to conventional lung function testing done in the past.
1. INTRODUCTION
Chronic Obstructive Pulmonary Disease (COPD) is defined as a multicomponent, preventable, treatable, partially irreversible chronic inflammatory, respiratory disease with extra pulmonary manifestations. It is characterized by progressive and persistent airflow limitation, usually resulting from the chronic inflammatory reaction of smaller airways due to tobacco smoking and other inhaled toxins [1, 2]. COPD has different phenotypes and is believed to encompass a spectrum of diseases, such as “emphysema,” destruction of gas-exchange area of alveoli at one end, and “chronic bronchitis” at the other, which may precede or follow COPD [3, 4]. This disease, unfortunately, remains underdiagnosed or misdiagnosed since most of the time, it is considered to be the same airway obstruction that is seen in bronchial asthma. COPD and asthma can easily be differentiated by the variability of the airflow obstruction in asthma due to the hypersensitive allergic response of airways to any foreign agent (i.e., exacerbations). COPD is a chronic disease with slow progression, and obstruction remains in the stable state markedly over several months. Although the same structural changes are seen in both ailments, there are some obvious differences in airway functioning [5]. COPD can be classified as stable or exacerbated COPD on the basis of disease severity, clinical presentation, and lung function tests by spirometry [6, 7]. Cigarette smoking is the strongest risk factor in the development of COPD worldwide, and the current knowledge on COPD and its associated morbidity and mortality comes from those analyses which are made in smoking-related COPD [8,9]. The smoking burden is usually measured in pack-year, and to calculate the pack-year, smokers are asked to report the average number of cigarettes smoked per day in the duration in years. One pack-year means smoking an average of one pack (20 cigarettes) every day for one complete year [10]. The pack-years calculation assumes the same weightage for both duration of smoking and cigarettes/day though the relative contribution of the duration of smoking versus cigarettes/day toward COPD is not known [11]. The current strategy to diagnose COPD on clinical grounds or additionally by the measurement of respiratory functions by spirometry potentially leaves many patients underdiagnosed and sometimes over-diagnosed. Globally, 10 – 95% under-diagnosis and 5 – 60% over-diagnosis of disease are prevalent due to the unavailability of spirometry in rural areas of the developing countries, untrained technical operators to perform the test, and their lack of skill in interpretating the results of spirometry [12,13]. The diagnosis of COPD can only be declared if patients have a combination of certain symptoms, “a post-bronchodilator fixed ratio of Forced Expiratory Volume in 1st second and Forced Vital Capacity, i.e., FEV1/FVC < 0.7, measured by spirometry [14]. Hence in the absence of any reliable and accurate diagnostic criteria, there is a dire need to search for some authenticated markers for the diagnosis of COPD. Effective therapeutic interventions are not possible without a proper and accurate diagnosis of any disease. Conversely, an overdiagnosis or false-positive diagnosis may also cause the true underlying diagnosis to be missed. Therefore, it has been hypothesized that to diagnose COPD and its clinical progression, biomarkers should be searched as a suitable substitute for previously used diagnostic tools [15]. Various body compartments like breath condensate, blood, sputum, bronchoalveolar lavage, and urine as samples are tested for the detection of biomarkers. CCL-18, Surfactant protein-D (SP-D), CC-16 (Clara cell protein-16), MMPs 8 and 9 (matrix metalloproteinase), IL-6 and 8 (Interleukin), and CRP (C-reactive protein) are distinguishable blood biomarkers which are considered to be the potential diagnostic markers for COPD, but none of them has been proved to be significant in this context [16, 17]. Amongst all other biomarkers under consideration, fibrinogen is found to play a pivotal role in diagnosing COPD. Fibrinogen is glycoprotein, which is synthesized in hepatocytes and serves as an essential coagulation factor when released into blood circulation. In COPD, the inflammatory role of fibrinogen as an acute-phase reactant makes it an ideal biomarker [18, 19]. Fibrinogen, an inflammatory marker, is found to be increased not only due to coagulation but also following a tissue injury. The plasma levels of fibrinogen proved to be a predictor of acute exacerbations of COPD as well [20]. Plasma fibrinogen levels, along with other factors like blood cells, may predict the frequency of the attack and clinical phenotype of COPD; frequency of exacerbations of disease is also determined by these biomarkers [21].
The comprehensive meta-analysis of different observational studies though proved the hypothesis of circulating fibrinogen as a biomarker, but still, nearly all qualified studies are measuring the circulating fibrinogen concentration only once, and there is no long-term change evaluation in the development of COPD. Furthermore, measurement bias is also suspected because no identical method is used to assay circulating fibrinogen across studies. Thereby, future well-performed studies on larger scales are suggested for drawing a conclusion, and for refusing or confirming our findings [19].
In almost all components of the disease (progression, severity, associated co-morbidities, mortality, diagnosis, and treatment strategies), plasma fibrinogen levels show a significant correlation with COPD. An inverse relationship was found between baseline fibrinogen levels and pulmonary functions, i.e., if fibrinogen is higher than normal at the beginning of COPD, declined FEV1 and FVC will be observed over the course of the disease [22]. This difference was observed in current smokers and non-smokers as well. Moreover, higher baseline fibrinogen levels were also associated with an increased incidence of admissions with an exacerbation of COPD. It has been studied that there is a promising relationship between raised plasma fibrinogen at baseline in individuals with COPD and the development of frequent exacerbations of disease and hospitalization as well [23].
2. METHODOLOGY
A comparative cross-sectional study was conducted in the Physiology Department of Sheikh Zayed Postgraduate Medical Institute, Sheikh Zayed Hospital Pulmonology Department, Lahore, in collaboration with the Combined Military Hospital. The total 75 patients were divided into 3 groups (controls = 25, exacerbated = 25 and stable COPD = 25 patients). Gender matched, young adults (20 - 39 years of age) to old aged (60 - 80 years of age) individuals with a history of smoking at least 10 pack-years (20 cigarettes/day for 1 complete year = 1 pack-year) were included in the three groups. All the normal and healthy individuals without a history of COPD were included as controls. Patients diagnosed with COPD according to GOLD criteria, having a history of smoking of more than 10 pack-years (20 cigarettes/ day for 1 complete year = 1 pack year) and a state of exacerbation free period of 3-4 months, were enrolled as stable cases. Patients with diagnosed COPD in an exacerbated state, having a history of acute attacks in the last few days to 1 week,presented in the emergency department or already hospitalized, were enrolled as exacerbated cases. The exclusion criteria included diagnosed cases of asthma or other chronic respiratory diseases on the basis of spirometric findings, patients with history of malignancy or serious co-morbidities that would prevent the study completion and patients diagnosed with active pulmonary tuberculosis or bronchiectasis due to old complicated TB. The study was started after the approval from the Ethical Review Board of Federal Post Graduate Medical Institute Sheikh Zayed Medical Complex Lahore. Data including name, age, weight, height, history of smoking, and other diseases in the past were recorded through a questionnaire. A detailed present and past history were recorded, and a physical examination was performed. A 6-minute walk test was performed to measure the grading of dyspnoea on all the participants except those who were unable to take a single step due to shortness of breath (exacerbated COPD cases). After observing all aseptic measures, 3cc of blood was obtained from the cubital vein using venepuncture in 3.2% sodium citrate containing vial. The plasma was separated from blood by centrifugation at the rate of 5000 revolutions per minute for 10 minutes. It was then stored in an aliquot and kept frozen at -20C till the required test was performed. The test was then conducted by indirect coagulation clauss method in haematology laboratory of Combined Military Hospital Lahore after due permission from the authorities. A human Hemostat fibrinogen kit was used for the estimation of fibrinogen levels
The obtained data was entered and analysed by using SPSS 22.0. The data for quantitative variables, i.e., age, BMI, smoking pack years, FEV1, FEV1/FVC, FEV1 pp, fibrinogen levels in blood and the qualitative variables, i.e., grading of dyspnoea and gold staging of COPD were described by using descriptive statistics, i.e., mean ± SD for the three groups. The comparison of these variables among groups was studied by using one-way ANOVA; p-value of ≤ 0.05 was considered significant.
3. RESULTS
In control group, the majority of the patients were adults (12 (80%)) and middle-aged (11 (44%)), having normal weight (13 (52%)) and smoking 10 - 12 packs of cigarettes per year (19 (76.0%)). In the exacerbated group, the majority of the patients were middle aged (17 (68%)), having normal BMI (16 (64%)) and smoking 21 - 40 packs of cigarette per year. In the stable COPD group, the majority of the patients were old aged (12 (16%)), having normal BMI (18 (72%)) and smoking 10 - 12 packs of cigarette per year (Tables 1.1-1.3).
| Groups | |||
| Variables | Control | Exacerbated | Stable |
| Adults (15 - 40) | 12 (80%) | 1 (4%) | 8 (32%) |
| Middle aged (41 - 60) | 11 (44%) | 17 (68%) | 5 (20%) |
| BMI | ||
| Underweight | 4 (12%) | 4 (16%) |
| Normal | 13 (52%) | 16 (64%) |
| Overweight | 8 (32%) | 5 (20%) |
| Smoking Status | |||
| 10 - 12 Packs per year | 19 (76.0%) | 8 (32%) | 17 (68%) |
| 21 - 30 Packs per year | 4 (16%) | 6 (24%) | 6 (24.0%) |
| 31 - 40 Packs per year | 2 (8%) | 10 (40%) | 1 (4.0%) |
| > 40 Packs per year | 0 (0%) | 1 (4.0%) | 1 (4.0%) |
3.1. Clinical History of Patients
According to Tables 2.1-2.4, in the control group, the majority of the patients had grade 2 dyspnea, low FEV1 (Below 3 L) (16 (64%)) and normal FEV/FVC (24 (96%)). In the exacerbated group, the majority of the patients had grade 4 dyspnea (21 (84%)), stage 3 COPD 15 (60%), very low (Below 1 L) (21 (84%)) and low FEV1/FVC (19 (76%)). In the stable COPD group, the majority of the patients had G3 dyspnea (19 (76%)), stage 1 of COPD (18 (72%)), FEV1 Low (Below 2 L) (19 (76%)) and Very low FEV1/FVC (20 (80%)).
| Grading of Dyspnea | |||
| G1 | 7 (28%) | 0 (0%) | 0 (0%) |
| G2 | 16 (64%) | 1 (4%) | 4 (16%) |
| G3 | 2 (8%) | 3 (12%) | 19 (76%) |
| G4 | 0 (0%) | 21 (84%) | 2 (4%) |
| Stages of COPD | |||
| Stage 1 | 0 (0%) | 0 (0%) | 18 (72%) |
| Stage 2 | 0 (0%) | 5 (20%) | 3 (12%) |
| Stage 3 | 0 (0%) | 15 (60%) | 4 (16%) |
| Stage 4 | 0 (0%) | 5 (20%) | 0 (0%) |
| Forced Expiratory Volume in 1 second (FEV1) | |||
| Normal (3 - 4 L) | 7 (28%) | 0 (0%) | 0 (0%) |
| Low (Below 3 L) | 16 (64%) | 1 (4%) | 4 (16%) |
| Low (Below 2 L) | 2 (8%) | 3 (12%) | 19 (76%) |
| Very Low (Below 1 L) | 0 (0%) | 21 (84%) | 2 (8%) |
| FEV1/FVC | |||
| Normal (> 80) | 24 (96%) | 1 (4.0%) | 0 (0.0%) |
| Low (< 80) | 1 (4.0%) | 19 (76%) | 5 (20%) |
| Very low (< 60) | 0 (0.0%) | 5 (20%) | 20 (80%) |
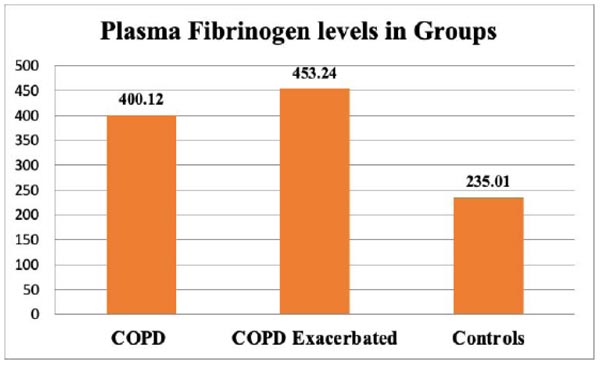
Plasma fibrinogen levels were compared among the three groups. Mean ± SD of plasma fibrinogen levels were 453.24 ± 81.92 mg/dl in the exacerbated COPD group, 400.12 ± 81.45 mg/dl in the stable COPD group, and 235.01 ± 45.015 in the healthy controls. It was seen that plasma fibrinogen levels were raised in patients in an exacerbated state of COPD and also in patients having stable COPD (p-value = 0.000) (Fig. 1).
4. DISCUSSION
The aim of this research work was to determine a diagnostic biomarker for a misdiagnosed and consequently wrongly treated disease of COPD; for this purpose, plasma fibrinogen was considered a surrogate endpoint biomarker. The association of plasma fibrinogen levels with COPD and its severity was examined in 75 individuals who were smokers, and 50 of them were confirmed patients of COPD, diagnosed by their reduced pulmonary functions. The key finding of this experimental work suggests that a significant, concentration-dependent relation exists between COPD and its increasing severity and higher circulating fibrinogen. Fibrinogen in the human body is a plasma protein synthesized in the liver mainly; it is converted into fibrin during blood coagulation [24]. It has been observed that fibrinogen synthesis is up-regulated as a major acute-phase reactant in response to inflammation, and this is a major clinical feature of COPD [25]. In addition, evidence also exists that the risk of exacerbation of COPD is increased with elevated fibrinogen plasma [26]. Furthermore, fibrinogen concentration is correlated with impaired lung functions and is related to increased mortality among patients with COPD [27]. Plasma fibrinogen is hence reasonably speculated as a promising clinical biomarker in predicting the risk and severity of COPD [28]. Based on various meta-analyses, a significant relationship between severity of COPD and circulating fibrinogen has been established [29-34]. Another important fact is a possible interaction between cigarette smoking and plasma fibrinogen. Amongst the established risk factors for the development of COPD, cigarette smoking and its contribution to COPD disease is interestingly found to be mediated by elevated plasma fibrinogen, which though could not be reliably investigated in the present study due to the unreliable history of individual participants of the study, yet we agree that further explorations on the fact are needed. Importantly, plasma fibrinogen is a biomarker that is easy to assay and can be easily proposed as a more practical and useful approach toward clinical management of COPD. There are certain limitations of the study; firstly, it is a cross-sectional study with a small sample size, therefore, there are fair chances that it might have affected the analysis of statistical power of the results. Multiple prospective and extensive studies on COPD patients have been conducted worldwide, but still, in Asians, no significant research has been done. Secondly, plasma fibrinogen concentration was only measured once, therefore, we could not evaluate the long-term effects of the change of fibrinogen on the progression of COPD. Thirdly, the usefulness of fibrinogen levels could not be investigated for predicting mortality with COPD. Thereby, drawing a conclusion is not possible until some longitudinal, large-scale, and well-performed studies confirm or refuse the results of our study.
CONCLUSION
The plasma inflammatory biomarker profile, identified in patients with COPD in a stable and exacerbated state, represents a valuable tool for the replacement of lung function testing in the assessment and clinical presentation of COPD. The need for biomarkers to identify the heterogeneity of COPD and characterize and continuously improve the identification of disease progressors is mandatory for the efficient management of the disease. Our study is the first research on the population of Pakistan to consider plasma biomarker fibrinogen level as a significant indicator for COPD. We have concluded that easy to assay, plasma fibrinogen levels may serve as a useful marker for earlier detection of the potential causes of COPD. If measured prospectively during close follow-up, fibrinogen levels can serve as a prognostic marker as well. Consequently, plasma fibrinogen will help in improving the clinical outcome and for the effective treatment of COPD.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has been approved by the institutional review board of the 'Federal PostGraduate Medical Institute, Sheikh Zayed Hospital National Health Research Complex Lahore, Pakistan. (Registration number: Institutional Review Board, Ref No: F-39/NHRC/Admin/IRB/86 IRB No:1274).
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Patients were informed about this study.
AVAILABILITY OF DATA AND MATERIALS
The data supporting this study is present within the manuscript.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


