All published articles of this journal are available on ScienceDirect.
Significance of Carbohydrate Antigen 19-9 as a biomarker in Hepatocellular Carcinoma and Cholangiocarcinoma
Abstract
Background & Aims:
Combined hepatocellular - cholangiocarcinomas (cHCC-CCs) are rare malignancies representing less than 1% of all primary liver cancers. Correct preoperative diagnosis is desirable because the frequency of lymph node metastasis in ICC and cHCC-CC, making lymph node dissection a necessity if curative resection to be attempted. This study aimed to investigate the significance of elevated CA19-9 in suspecting a diagnosis of Intrahepatic Cholangiocarcinoma (ICC) “non-invasively” in patients with typical radiological features of HCC.
Methods
This cross-sectional study was conducted on 54 patients with typical radiological criteria of HCC and elevated CA19-9 level. And were classified into two groups I included 22 patients (40.74%) who were diagnosed as HCC, group II included 30 patients (55.56%) were diagnosed as ICC, and there were 2 patients (3.7%) were diagnosed as cHCC-CC.
Tumor markers (AFP and CA19-9), dynamic study (Triphasic CT or Dynamic MRI) were done for all patients. Target liver biopsy was done for histopathology and immuno-histochemistry using specific monoclonal antibodies against Glypican-3, Hep-par1, CK-7, CK-19 and CK-20 were done.
Results:
There was a statistically significant difference between HCC and ICC as regard CA19-9 and Alpha-fetoprotein (AFP). CA19-9 and AFP cut-offs were ˃ 58.9 U/mL and ˂ 25.8 ng/mL, respectively favoring the diagnosis of ICC, with very high sensitivity and specificity. CA19-9 level was 176.3 and 156.7 U/mL while AFP level was 460 and 170 ng/mL in cHCC-CC cases, respectively.
Conclusion:
CA19-9 could be a diagnostic marker of ICC in cases of typical radiological criteria of HCC with elevated CA19-9.
1. INTRODUCTION
According to the WHO, HCC is the fifth most common cancer and the second most frequent cause of cancer-related death globally [1]. HCC represents about 90% of primary liver cancers [2].
In 2012, Egypt had a high incidence rate. HCC is the first most common cancer in men and the second most common cancer in women [3].
ICC is the second most common primary hepatic malignancy (10-20%) after HCC and accounts for about 3% of all gastrointestinal cancers [4].
ICCs are large tumors and the presence of lymph node metastasis is associated with poor outcomes[5 . Also, ICC has been considered a contraindication for (LT) [6]. Moreover, even patients found to have an incidental ICC have poor transplant outcomes [7].
Combined HCC-CCs are rare malignancies, representing less than 1% of all primary liver cancers [8].
Approximately 1% of lesions presumed to be HCC based on imaging characteristics will turn out to be cHCC-CC on the final explant pathology after LT [9]. The clinical characteristics of cHCC-CC were similar to those of HCC [10], but overall survival was more similar to or poorer than that of CCA [11].
Sensitivity of both CT and MRI imaging for detecting cHCC-CC was about 33%, with lesions being mistaken for both HCC and CC; thus highlighting the diagnostic challenges associated with this diagnosis [12].
The definite diagnosis of cHCC-CC can be made by histopathological examination only along with the use of Immunohistochemistry (IHC) [13].
Correct preoperative diagnosis is desirable because of the high frequency (70%) of lymph node metastasis, making lymph node dissection a necessity for curative resection [14].
LT has no role and is not a good management option for cHCC-CC [15]. Most of the data on transplanted cHCC-CC patients came from patients that were initially misdiagnosed with HCC [16]. Outcomes are worse when compared with HCC due to associated higher recurrence rates after transplant [17].
So, This study aimed to investigate the significance of elevated CA19-9 in suspecting a diagnosis of Intrahepatic Cholangiocarcinoma (ICC) “non-invasively” in patients with typical radiological features of HCC.
2. MATERIALS AND METHODS
The study protocol was performed according to the ethical guidelines of the Helsinki Declaration and was approved by the ethical committee of Benha University Faculty of Medicine. Written informed consent was signed by all patients participating in the study.
- This cross-sectional observational study was conducted on 54 patients with typical radiological criteria of HCC and elevated CA19-9 level, who attended to Hepatology, Gastroenterology, and infectious disease and internal medicine departments in Benha University Hospital, Benha University and Intervention Radiology Unit at National Hepatology and Tropical Medicine Research Institute (NHTMRI); Cairo, within the period between October 2017 and April 2019.
- They were divided into two groups: group (1) included 22 patients who were diagnosed as HCC, group (2) included 30 patients who were diagnosed as ICC. And there were two other patients who were diagnosed as cHCC-CC.
- All studied subjects underwent the following:
2.1. A Detailed History Taking, Thorough Clinical Examination
2.1.1. Biochemical Investigations
Complete blood count, transaminases, bilirubin level, serum albumin, INR and renal functions.Viral markers (HCV-Ab and HBsAg by ELISA test). Tumor markers (AFP and CA19-9; by an RIA test using the “ADVIA CENTAUR SIEMENS” system).
2.1.2. Imaging
Abdominal ultrasound (using iU22 xMatrix -DS Ultrasound system from PHILIPS with liver scanning protocol using a curved array PureWave transducer), and dynamic study (triphasic CT or dynamic MRI).
2.1.3. Histopathological Examination
Target liver biopsy was done using ultrasound-guided percutaneous technique; using 18-gauge core biopsy needle, and the collected liver mass tissue was put into 10% formalin immediately after the procedure and the paraffin blocks were prepared from the collected liver mass tissue specimen, and the sections were stained with Hematoxylin and Eosin (H&E) for histopathological examination.
2.1.4. Immuno-histochemistry Examination
Sections were prepared from the paraffin blocks then treated by using specific monoclonal antibodies against Glypican-3 (GPC3), (Hepatocyte Paraffin-1), Hep-par1, (Cytokeratin 7) CK7, (Cytokeratin 19) CK19, and (Cytokeratin 20) CK20. Automated B-Link 48 system from Dako with FLEX kit was applied. DAB was applied as chromogen and hematoxylin as counter-stain for immuno-histochemistry examination. Positive staining by GPC3 and Hep-par1 was consistent with the HCC component [18, 19], while positive staining by CK7 and CK19 was consistent with the CCA component, but with negative staining by CK20, which is specific for extrahepatic CCA [20, 21].
2.2. Statistical Analysis
Patients' data were analyzed using Statistical Program for Social Science (SPSS) version 15.0 for windows. Quantitative data were expressed as mean ± Standard Deviation (SD) and median. Qualitative data were expressed as frequency (No.) and percentage (%). Chi-square test (X2) was used when comparing non-parametric data. P-value was set at ≤ 0.05 for significant results and > 0.05 for insignificant results. ROC curve was used to determine cut-off value, and the following statistics can be defined: sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
3. RESULTS
The fifty-four (54) patients were chosen from 622 patients with typical radiological criteria of HCC, for them CA19-9 was done and who showed elevated level were included in this study, while patients with atypical radiological features of hepatocellular carcinoma, patients with negative CA19-9, and patients with ascites were excluded from the study.
The studied patients were classified into two groups based on the pathological diagnosis. 22 patients (40.74%) were diagnosed as HCC, 30 patients (55.56%) were diagnosed as ICC, and the two patients were diagnosed as cHCC-CC.
As regards the 2 cHCC-CC patients, They were 58 and 45 years old males; one of them gave symptoms of liver cirrhosis, but none of them showed signs of liver cell failure during clinical examination and laboratory assessment. In both cases, serology was positive for HCV and negative for HBV.
AFP level was 460 and 170 ng/mL, and CA19-9 level was 176.3 and 156.7 U/mL for both cases, respectively. Their abdominal ultrasonography revealed: Enlarged coarse liver, patent dilated PV, right lobe single mass, with not dilated CBD or IHBRs or ascites were noted. Their dynamic study revealed: Right lobe single mass showing the typical radiological criteria of HCC, associated with porta-hepatis Lymph Nodes (LNs) metastasis in both cases, but one of them showed more LNs metastasis to para-aortic and celiac LNs.
For both cases, a target liver biopsy was done and the histopathological result was HCC grade II mixed with carcinoma cells forming glandular structures and mucin production. On IHC, malignant cells were positive for GPC3, Hep-par1, CK7, and CK19, but were negative for CK20; these results were consistent with cHCC-CC.
As regards the rest of the patients (HCC and ICC groups); the mean age was 63.27 ± 8.76 years in HCC and 59.60 ± 9.79 years in ICC patients, and 63.6% of HCC and 73.3% of ICC patients were males, with no statistically significant difference (P-value was 0.16 and 0.45, respectively) (Table 1).
The socio-demographic characteristics showed no statistically significant difference as regards the residence and smoking state, 36.4% of HCC and only 6.7% of ICC patients were hypertensive; also 18.2% of HCC and 33.3% of ICC were diabetics, 63.6% of HCC and only 6.7% of ICC patients had esophageal varices, none of HCC and 26.7% of ICC patients had jaundice, 54.5% of HCC and only 20% of ICC patients had lower limb edema, with statistically significant difference (P-value was 0.007, <0.001, 0.008 and 0.01, respectively) (Table 1).
Mean Platelets (PLT) count was 175.55±78.09 thousands/Cmm in HCC and 300.87±105.57 thousand/Cmm in ICC patients, mean aspartate aminotransferase (AST) 102.14±97.55 U/L in HCC and 49.53±15.09 U/L in ICC patients, mean Alkaline Phosphatase (ALP) was 152.77±61.73 U/L in HCC and 345.27±198.81 U/L in ICC patients, mean prothrombin concentration (PC) was 80.78±15.3% in HCC and 88.88±12.83% in ICC patients, All HCC patients had HCV, and only 46.7% of ICC patients had HCV, there was a statistically significant difference (P-value was ˂0.001, 0.02, ˂0.001, 0.04 and ˂0.001, respectively) (Table 2), While hemoglobin level, leucocytic count, alanine transaminase (ALT), albumin, bilirubin, INR, creatinine, and urea levels, HBsAg showed no statistically significant difference between the 2 groups (Table 2).
| Variable |
Group I (HCC) (No.22) |
Group II (ICC) (No. = 30) |
P-value |
|---|---|---|---|
| Age(Years) mean± SD | 63.27± 8.76 | 59.60±9.79 | 0.16 |
| Sex | |||
| Male | 14(63.6%) | 22(73.3%) | 0.45 |
| Female | 8(36.4%) | 10(33.3%) | |
| Residence | |||
| Rural | 16(72.7%) | 20(66.7%) | |
| Urban | 6(27.3%) | 10(33.3%) | 0.64 |
| Smoking | |||
| Smoker | 6(27.3%) | 6(20%) | |
| ExSmoker | 2(9.1%) | 10(33.35) | 0.12 |
| DM | 4(18.2%) | 10(33.3%) | 0.22 |
| HTN | |||
| Yes | 8(36.45) | 2(6.7%) | |
| No | 14(63.6%) | 28(93.35) | 0.007(S) |
| Weight loss | |||
| Yes | 22(100%) | 30(100%) | |
| No | 0(0%) | 0(0%) | ---- |
| Jaundice | |||
| Yes | 0(0%) | 8(26.75) | |
| No | 22(100%) | 22(73.3%) | 0.008(S) |
| Ascites | |||
| Yes | 2(9.1%) | 6(20%) | |
| No | 22(90.9%) | 24(80%) | 0.28 |
| LL oedema | |||
| Yes | 12(54.4%) | 6(20%) | |
| No | 10(45.5%) | 24(80%) | 0.01(S) |
| Esophageal varices | |||
| Yes | 14(63.6%) | 2(6.7%) | |
| No | 8(36.4%) | 28(93.35) | 0.001(HS) |
| Bleeding tendency | |||
| Yes | 3(13.65) | 4(13.35) | |
| No | 19(86.4%) | 26(86.7%) | 0.97 |
| Variables |
Group I (HCC) (No. = 22) |
Group II (ICC) (No. = 30) |
P-value |
|---|---|---|---|
| Hb (gm/dL) Mean± SD | 12.50± 1.87 | 12.61±1.53 | 0.8 |
| WBCs (thousands/Cmm) Mean± SD | 8.33±4.12 | 8.48±4.18 | 0.89 |
| Platelets(thousands/Cmm) Mean± SD | 175.55±78.09 | 300.87±105.57 | <0.001 |
| ALT (U/L) median | 41 | 46.5 | 0.92 |
| AST(U/L) median | 68 | 49 | 0.02(S) |
| S. Albumin (gm/dL) Mean± SD | 3.37±0.44 | 3.57± 0.47 | 0.12 |
| Alkaline phosphatase(U/L)Mean± SD | 152.77±61.3 | 345.27±108.81 | 0.04 |
| Prothrombin concentration(PC) Mean± SD | 80.78±15.3 | 88.88±12.83 | <0.001 |
| T. Bilirubin (mg/dL) Mean± SD | 1.26± 0.37 | 1.48± 0.81 | 0.23 |
| D. Bilirubin (mg/dL) Mean± SD | 0.65±0.37 | 0.71±0.64 | 0.68 |
| INR Mean± SD | 1.22±0.18 | 1.16±0.24 | 0.37 |
| Creatinine (mg/dL) Mean± SD | 0.96±0.21 | 1.00±0.27 | 0.5 |
| Urea (mg/dL) Mean± SD | 39.97±11.65 | 34.02 ±12.51 | 0.08 |
| HBsAg (-ve) | 22(100%) | 30(100%) | -- |
| HCV Ab | |||
| Yes | 22(100%) | 14(46.75) | |
| No | 0(0%) | 16(53.3%) | 0.001 |
| AFP(ng/ml) median | 36.3 | 8.8 | 0.003(S) |
| CA19-9(u/ml) median | 65.5 | 54.8 n | 0.004(S) |

Median AFP was 36.3 ng/mL in HCC and 8.8 ng/mL in ICC patients, median CA19-9 was 54.8 U/mL in HCC and 165.5 U/mL in ICC patients, with statistically significant difference (P-value was 0.003 and 0.004, respectively) (Table 2).
The findings of the dynamic radiological study (triphasic CT and dynamic MRI) and abdominal ultrasonography, including the number and site of masses, showed no statistically significant difference (Table 3).
None of the HCC patients had LNs metastasis, while 6.7% of ICC patients had porta-hepatis LNs. metastasis and 20% had para-aortic and celiac LNs metastasis, there was a statistically significant difference (P-value was 0.031) (Table 3).
On histopathological base, results of HCC patients; 63.6% were HCC grade I, 27.3% were HCC grade II, and 9.1% were HCC grade III. And as regard ICC patients; 6.7% were moderately differentiated adenocarcinoma, 60% were poorly differentiated adenocarcinoma and 33.3% were undifferentiated malignant tumor (Table 4).
Based on IHC; all cases of HCC showed positive staining for GPC3 and Hep-par1 and negative staining for CK7, CK19 and CK20, while all cases of ICC showed positive staining for CK7 and CK19 and negative staining for GPC3, Hep-par1, and CK20, there was a statistically significant difference (P-value was ˂0.001) (Table 5).
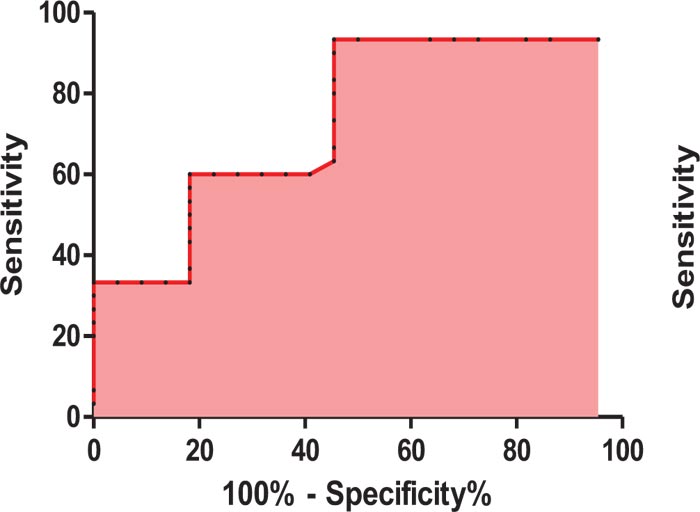
ROC curve analysis showed that CA19-9 can be used in discrimination between HCC and ICC patients at a cut off level of > 58.9 U/mL, with 93.3% sensitivity, 54.5% specificity, 67.2% PPV and 89.05% NPV with an AUC 0.73 (P-value was 0.003) (Table 6, Fig. 1).
While ROC curve analysis of AFP showed that AFP could be used in discrimination between HCC and ICC patients at a cut off level of < 25.8 ng/mL, with 86.7% sensitivity, 63.6% specificity, 70.43% PPV and 82.7% NPV with an AUC 0.73 (P-value was 0.003) (Table 7, Fig. 2).
Table 3.
| Variables |
Group I (HCC) (No. = 22) |
Group II (ICC) (No. = 30) |
P-value |
|---|---|---|---|
| Mass No. | |||
| Single | 15(68.2%) | 20(66.7%) | 0.908 |
| Multiple | 7(31.8%) | 10(33.3%) | |
| Mass site | |||
| center lobe | 3(13.6%) | 0(0%) | |
| Right lobe | 10(45.5%) | 14(46.7%) | 0.105 |
| Bi-lobar | 9(40.9%) | 16(53.3%) | |
| L.N. metastasis | |||
| Negative | 22(100%) | 22(73.3%) | |
| Porta-hepatis | 0(0%) | 2(6.7%) | 0.31(S) |
| Para-aortic and celiac | 0(0%) | 6(20%) | |
|
Mass echogenicity (by U/S) |
|||
| Isoechoic | 2(9.1%) | 2(6.7%) | |
| Hypoechoic | 9(40.95) | 15(50%) | 0.8 |
| Heterogeneous | 11(50%) | 13(43.3) |
| No | % | |
|---|---|---|
| Group I (HCC) | ||
| HCC grade I | 14 | 63.6 |
| HCC grade II | 6 | 27.3 |
| HCC grade III | 2 | 9.1 |
| Group II (ICC) | ||
| Moderately differentiated adenocarcinoma | 2 | 6.7 |
| Poorly differentiated adenocarcinoma | 18 | 60 |
| Undifferentiated malignant tumor | 10 | 33.3 |
| Variables |
Group I (HCC) (No. = 22) |
Group II (ICC) (No. = 30) |
P-value |
|---|---|---|---|
| Glypican-3 | |||
| Negative | 0(0%) | 30(100%) | < 0.001(S) |
| Positive | 22(%) | 0(0%) | |
| Hep-par1 | |||
| Negative | 0(0%) | 30(100%) | < 0.001(S) |
| Positive | 22(100%) | 0(100%) | |
| CK7 | |||
| Negative | 22(100%) | 0(0%) | < 0.001(S) |
| Positive | 0(100%) | 30(100%) | |
| CK19 | |||
| Negative | 22(100%) | 0(0%) | < 0.001(S) |
| Positive | 0(100%) | 30(100%) | |
| CK20 | |||
| Negative | 22(100%) | 30(100%) | ------- |
| Positive | 0(100%) | 0(100%) |
| Cut off | Area under the curve | Sensitivity | Specificity | PPV | NPV | P-value |
|---|---|---|---|---|---|---|
| > 58.9 U/mL | 0.73 | 93.3% | 54.5% | 67.2% | 89.05% | 0.003 S |
| Cut off | Area under the curve | Sensitivity | Specificity | PPV | NPV | P-value |
|---|---|---|---|---|---|---|
| < 25.8 ng/mL | 0.73 | 86.7% | 63.6 | 70.43% | 82.7% | 0.003 S |
4. DISCUSSION
This study was conducted on 54 patients with typical radiological criteria of HCC and elevated CA19-9 aiming to investigate its significance in suspecting a diagnosis of cHCC-CC.
Two patients were diagnosed as cHCC-CC with an incidence 0.32% from all primary liver tumors in patients who visited the NHTMRI during the study period. And this was in agreement with Wang et al., 2016 [8] who reported combined HCC-CC incidence accounts for 0.4 - 14.2% of primary liver cancer cases.
They were 58 and 45 years old males. Both cases serology was positive for HCV and negative for HBV and this was in agreement with Jarnagin et al., 2002 [22] who reported similar patient profile as regard strong male predominance, associated underlying cirrhosis (40%) and hepatitis (70%) between cHCC-CC and HCC patients.
AFP level was 460 and 170 ng/mL, and CA19-9 level was 176.3 and 156.7 U/mL for both cases, respectively. Their dynamic study revealed: Right lobe single mass showing the typical radiological criteria of HCC, associated with porta-hepatis LNs metastasis in both cases, but one of them showed more LNs metastasis to para-aortic and celiac LNs, and this was in agreement with O'Connor et al., 2014 [23] who reported the combination of elevated CA19-9 and AFP or radiological criteria of HCC should alert investigators for the possibility of cHCC-CC.
For both cases, a target liver biopsy was done and the histopathological result was HCC grade II mixed with carcinoma cells forming glandular structures and mucin production was noted, and this was in agreement with Theise et al, 2010 [24] who reported differentiated hepatocellular and biliary components in the tumor is required for histopathological diagnosis of cHCC-CC.
On IHC, malignant cells were positive for GPC3, Hep-par1, CK7 and CK19, but were negative for CK20; these results were consistent with cHCC-CC, and this was in consistent with Yeh, 2010 [13] and O'Connor et al., 2014 [23] who reported the same IHC for both malignant components in cases of cHCC-CC, and Gigante et al., 2019 [25] who reported the same personal, laboratory, radiological, histopathological and IHC profile of cHCC-CC patients.
Regarding other patient groups (HCC and ICC groups), history of esophageal varices was more common in HCC than ICC patients (63.6% and only 6.7%, respectively), with a statistically significant difference between the two groups, and this was in agreement with Iavarone et al., 2016 [26] who reported that esophageal varices were common (59%) in HCC patients, and Gigante et al., 2019 [25] who reported only 5% of ICC patients had portal hypertension.
Jaundice was predominant in ICC (26.7%) than HCC patients, with statistically significant difference between the two groups, and this was in agreement with Li et al., 2014 [27] who reported predominance of jaundice in ICC patients, and Meng et al., 2014 [28] who reported very few cases of HCC has jaundice, and bile duct invasion was suspected in that study.
Hepatomegaly in our study was more common in ICC than HCC patients (33.3% and only 9.1%, respectively), splenomegaly was predominant in HCC (27.3%) than ICC patients, there was a statistically significant difference between the two groups, and this was similar with Reuben, 2016 [29] who reported primary liver tumors as a cause of hepatomegaly and splenomegaly.
There was a statistical significant decrease in PLT count in HCC than ICC patients (mean was 175.55±78.09 and 300.87±105.57 thousands/Cmm, respectively) and this was in agreement with Hsieh et al., 2017 [30] who reported mild thrombocytopenia in HCC patients, and Gigante et al., 2019 [25] who reported normal PLT count to mild thrombocytosis in ICC patients.
In the current study, all HCC patients had HCV and only 46.7% had in ICC patients, and this was in consistent with Mobarak et al., 2015 [31] who reported 100% of HCC patients had HCV in a study done at NHTMRI in 2015, Aljumah et al., 2016 [32] who reported that HCV was the most common underlying cause of HCC, and Sapisochin et al., 2014 [9] who reported only 42.9% of ICC patients had HCV.
As regards AFP it was significantly higher in HCC than ICC patients (median was 36.3 and 8.8 ng/mL, respectively), and this was similar with Hsieh et al., 2017 [30] who reported the median of AFP was 59.6 ng/mL in HCC patients, and Gigante et al., 2019 [25] who reported the median of AFP was 9.1 ng/mL in ICC patients.
CA19-9 was statistically significantly higher in ICC than HCC patients (median was 165.5 and 54.8 U/mL, respectively), and this was concordant with Lee et al.,2006 [33] who reported the median of CA19-9 was 56.1 U/mL in HCC patients, and Lumachi et al., 2014 [34] who reported the median of CA19-9 was 170.5 U/mL in ICC patients.
In this study, all HCC patients had coarse liver compared to 46.7% ICC patients who had coarse liver, and 53.3% had homogenous liver in ICC patients, and this was in agreement with Mobarak et al., 2015 [31] and Li et al., 2016 [10] who reported 99.3% of HCC and 39.9% of ICC patients had coarse cirrhotic liver, respectively.
All patients had patent PV but dilated PV was more common in HCC than ICC patients (54.5% and only 13.3%, respectively), and this difference was statistically significant between the two groups, and was matched with the study by Ruzzenente et al., 2011 [35] and Gigante et al., 2019 [25] who reported 32% of HCC and only 5% of ICC patients had portal hypertension, respectively.
In the current study, lymph nodes metastasis was statistically significantly predominant in ICC patients (6.7% had porta-hepatis LNs metastasis and 20% had para-aortic and celiac LNs metastasis compared to none of HCC patients had LNs metastasis), Ren et al., 2018 [36] and Wakizaka et al., 2019 [37] reported predominant LNs metastasis in ICC patients.
As regard histopathological results of HCC patients; 63.6% were HCC grade I, 27.3% were HCC grade II and 9.1% were HCC grade III, and this was in agreement with Zhang et al., 2014 [38] who reported similar pathological grading in HCC cases. While histopathological results of ICC patients, 6.7% were moderately differentiated adenocarcinoma, 60% were poorly differentiated adenocarcinoma and 33.3% were undifferentiated malignant tumor Sapisochin et al., 2014 [9] and Chen et al., 2017 [39] reported similar pathological differentiation in ICC patients.
regarding IHC; all cases of HCC showed positive staining for GPC3 and Hep-par1 and negative staining for CK7, CK19 and CK20, while all cases of ICC showed positive staining for CK7 and CK19 and negative staining for GPC3, Hep-par1 and CK20, this was in consistent with Ryu et al., 2012 [40] comparative study who reported similar results and differences between HCC and ICC cases.
Using ROC curve analysis, it was shown that CA19-9 at a cut off level of > 58.9 U/mL, could be used in discrimination between HCC and ICC patients, with 93.3% sensitivity, 54.5% specificity, 67.2% PPV, and 89.05% NPV; area under the curve was 0.73 and P-value was 0.003 denoting good predictive value of CA19-9 in the prediction of ICC, and this was in agreement with Leelawat et al., 2011 [41] and Kraiklang et al., 2014 [42] who reported that CA19-9 at a cut off level of 100 U/ml could be diagnostic for CCA, also was in agreement with Li et al., 2015 [43] who reported that, CA19-9 at a cut off level of ˃ 125.07 U/mL, could be helpful in the diagnosis of CCA with 76.67% sensitivity and 80% specificity.
Regarding ROC curve analysis of AFP, it was shown that AFP at a cut off level of < 25.8 ng/mL, can be used in discrimination between HCC and ICC patients, with 86.7% sensitivity, 63.6% specificity, 70.43% PPV and 82.7% NPV; area under the curve was 0.73 and P-value was 0.003 denoting the good predictive value of AFP in the prediction of ICC, and this was in concordant with Li et al., 2015 [43] who reported that, AFP cut off level of ˂ 15.4 ng/mL, with high sensitivity and specificity for the diagnosis of CCA when combined to CA19-9.
CONCLUSION
CA19-9 could be important in suspecting the diagnosis of the rare cases of cHCC-CC in patients with typical radiological criteria of HCC and elevated CA19-9 and, when combined with AFP, could be helpful in the diagnosis of pure cases of ICC in patients with typical radiological criteria of HCC.
LIMITATION OF THE STUDY
The sample size was limited. Therefore, large-scale studies are needed. Additional markers are urgently needed to be used
in order to improve the validity and reliability of findings.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work was approved by the Scientific Committee of Benha University Hospitals and the Institutional Review Board (IRB) of National Hepatology and Tropical Medicine Research Institute (NHTMRI); Cairo, with approval number Ms.5.2017.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was taken from each patient or relatives. Each patient received an explanation of the purpose of the study.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


