REVIEW ARTICLE
Phage Therapy in the Treatment of Infectious Diseases: An Overview
Mahsa Jalili1, 2, Nastaran Ansari1, 2, 3, Somaye Bakhtiari1, 2, Farid Azizi Jalilian1, 2, 3, *
Article Information
Identifiers and Pagination:
Year: 2021Volume: 11
First Page: 126
Last Page: 131
Publisher ID: TOBIOMJ-11-126
DOI: 10.2174/1875318302111010126
Article History:
Received Date: 13/3/2021Revision Received Date: 6/10/2021
Acceptance Date: 8/10/2021
Electronic publication date: 23/12/2021
Collection year: 2021

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Today, we are facing the spread of antibiotic resistance in various microbial communities. Also, researchers are using new methods to replace conventional treatments to prevent chronic bacterial infections. Hence, the used of phages or bacterial contaminant particles are now used as an effective method in the treatment of many infectious diseases. Several studies have suggested that the use of bacteriophages is effective in treating some bacterial diseases. Therefore, the present study was performed to evaluate phage therapy studies against infections caused by bacterial infections. The use of bacteriophages as new targets in the treatment of bacterial diseases restricts the development of infectious diseases. Bacteriophages can provide a new perspective in the development of new drugs to reduce the rate of bacterial infections. Also, it seems more research should be done in this field and more developed techniques should be used to evaluation of new phages.
1. INTRODUCTION
Bacteriophages were firstly introduced in 1886 by Hankin. Also, in 1931, two researchers, including Felix d'Hrelelle and George Eliava used bacteriophages to treat and prevent Vibrio cholera [1]. According to these documents, the first bacteriophages product against Vibrio cholerae was commercially produced in Georgia. Also, phage therapy was active from 1930 to 1940 in Georgia, Russia, Ukraine, Belarus and Azerbaijan [2]. In addition, in 1940, the Eli Lilly Institute produced seven phage products in the United States against bacterial infections, including Streptococcus, Staphylococcus, and Escherichia coli. On the other hand, the first study of phage therapy for septic infections was performed in 1940 at Ostroumovkaya Hospital in Moscow by intravenous and intramuscular injection of phage against septic infections [3]. Therefore, from 1939 to 1940, the Finnish campaign reported the use of phage produced by the Eliava Institute against Staphylococcus and Streptococcus to the improvement of wounds and surgery. In this regard, the discovery of penicillin by Alexander Fleming and the problems of phage therapy caused phage therapy to be forgotten for two decades [4]. So, the problems include a lack of sufficient knowledge in the field of phage biology and proper production techniques that led to the production of ineffective products. Therefore, despite all these problems, phage therapy continued in Eastern Europe [5].
On the other hand, the use of a wide range of antibiotics and the increase in resistance to them over the past two or three decades has created the challenge of the widespread antibiotic-resistant bacteria. Hence, widespread antibiotic resistance around the world has become a serious and important challenge [6]. So, antibiotic-resistant genes including beta-lactams, aminoglycosides, chloramphenicol and tetracycline are expanding in the type of bacteria family. Nowadays, multidrug resistance is one of the most challenging debates that microbiologists and physicians for the treatment of bacterial infections are facing [7]. Nowadays, various drugs were developed around the world to treat antibiotic resistance. Despite all the progress that was made in eradicating antibiotic resistance, it is almost impossible to discover drugs that can overcome the challenge of drug resistance [8]. Therefore, to overcome this problem, some methods such as the use of polymer compounds, nanoparticles, synthesis of new drugs, the use of proteomics and genomics methods were considered. In addition, one of the solutions suggested by microbiologists to reduce the damage caused by infectious diseases is to use herbal products and their derivatives as pharmacological products and anti-inflammatory drugs [9]. Furthermore, the presence of some properties such as antioxidant, antimicrobial, anti-inflammatory, and analgesic properties in herbal products has introduced them as a very good candidate to the pharmaceutical industry to limit bacterial infectious diseases [10]. On the other hand, various treatments were successful but in many cases, the side effects of these products are more than their therapeutic properties and lead to many problems. In addition, it seems that efficient and professional tools are needed to eliminate bacterial resistance [11]. Bacteriophages as new targets to limit bacterial growth could provide new perspectives on the development of new drugs to reduce the rate of bacterial infections. Hence, the present study was performed to use bacteriophages in the treatment and control of bacterial infectious diseases in a very key way and tries to provide a comprehensive perspective [12].
2. METHODS
The present study is a review research. So, various data in this study were collected by searching some keywords such as C.sativus, saffron, Iranian traditional medicine, medicinal properties, treatment of diseases in Persian and English in various search engines, as well as scientific databases such as PubMed and Science Direct; Articles and related texts were searched. Furthermore, findings and information were categorized through EndNote software and gradually organized. Finally, the article was written based on the findings.
3. LITERATURE REVIEW
3.1. Virology of Bacteriophages
Currently, twelve families of phage are considered for phage therapy against infectious diseases (Table 1). Thus, bacteriophages are divided into two groups: coated and uncovered. In addition, bacteriophages proliferation is similar to that of other viruses [13]. Also, bacteriophages attach to a specific receptor in the bacterial cell wall; after binding to a specific receptor in the bacterial cell wall, the phage genome enters the cell. The phage capsid coating remains on the bacterial cell wall and does not enter the bacterium. Also, after the bacteriophage enters the host cell, the phage genome is destroyed and degraded. Since then, an abundance of phages in the environment has been high. So bacteria have a mechanism to destroy the phage genome and prevent infection by these viruses. In addition, there are some loci in the phage genome; these loci are called restriction loci [14]. Therefore, bacterial enzymes can identify these areas and cut the phage genome in these areas. So, after phage genome cleavage, phage DNA is destroyed by bacterial enzymes. Also, as a result, the bacteria remain healthy and continue to cause infection and normal activity [15]. On the other hand, pathogenic bacteriophages replicate independently and destroy host cells. This contamination cycle is also called the bacteriophage lytic cycle. Therefore, these phages are used to kill bacteria and as therapeutic targets for infectious diseases (Fig. 1).
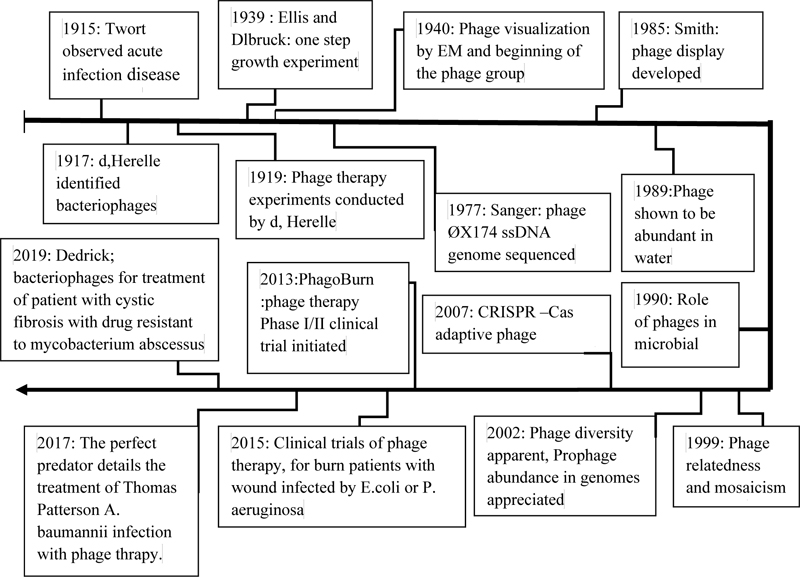 |
Fig (1). Important events related to phage and phage therapy research in recent years [19, 20]. |
| S.NO | Family | Gender | Example | |
|---|---|---|---|---|
| Enveloped bacteriophages | ||||
| 1 | Plasmaviridae | Plasmavirus | Acholeplasma phage | |
| 2 | Cystoviridae | Cystovirus | Ø6 | |
| 3 | Lipothrixviridae | Lipothrixvirus | Thermoproteus phage1 | |
| Non-Enveloped bacteriophages | ||||
| 1 | Inoviridae | Inovirus | Coliphage fd | |
| Plectrovirus | Acholeplasma phage | |||
| 2 | Leviviridae | Levivirus | Coliphage MS2 | |
| Allolevirus | Coliphage Qbeta | |||
| 3 | Microviridae | Microvirus | Coliphage ØX174 | |
| 4 | Spirovirus | Spiroplasma phage | ||
| 5 | Myoviridae | Coliphage T4 | ||
| 6 | Corticoviridae | Corticovirus | PM2 | |
| 7 | Podoviridae | Coliphage T17 | ||
| 8 | Siphoviridae | Lambda phage group | Coliphage lambda | |
| 9 | Sulpholobus shibatae virus | - | SSV-1 | |
| 10 | Tectiviridae | Tectivirus | Phage PRD1 | |
3.2. History of Phage Therapy
In 1919, bacteriophages were used to treatment of bacterial infections in Eastern Europe and the former Soviet Union. Nevertheless, in the west, phage therapy was not taken seriously [17]. In addition, in the west, a lack of knowledge about the biology of bacteriophages was the main reason for not using phage therapy. In 1915, bacteriophages were first observed in the bacterial environment by a British researcher. Also, in 1920, Dehler was able to identify the mechanism of phage science and report on the biological function of phages. So, in the 1920s and 1930s, the focus on phages in bacteriophage therapy programs peaked in the United States. In addition, an American company called Lily was able to produce a gel called Staphylococcusgel, which is labeled with bacteriophages. Furthermore, Abbott Laboratory succeeded in producing filters equipped with phage particles. Over the next years, researchers were able to discover the antibiotic properties of phages against bacterial infections [18].
In addition, between 1950 and 1980, many articles were published on phage therapy. Also, this trend continues from 2014 to 2016. Furthermore, with the keyword Phage therapy, more than 15,000 information records in the ISI appendix are available to users. Therefore, the high volume of information indicates the practical importance of phages in limiting infectious agents [19]. MedPage is one of the most important antibacterial drugs produced by pharmaceutical companies using phages to fight bacterial infections. Therefore, MediPhage is currently approved by the Food and Drug Administration (FDA) as an effective drug in limiting bacterial infections. Summary of the important events related to phage and phage therapy research on the length of time in (Fig. 1).
3.3. Commercial Phage Drugs
Currently, five groups of phages are used as appropriate targets in research laboratories in the treatment of bacterial infections. In addition, phages with brand names, Bacte-pyo-phage, Bacte-intesti-phage, Bacte-rhino-phage are available as effective phages in phage therapy [21]. Hence, Bacte-staphy-phage and Bacte-coli-phage are used against bacteria such as Escherichia coli, Streptococcus, Staphylococcus and other bacterial agents that cause infections in humans. Furthermore, some phage-derived compounds are produced to reduce the rate of infection caused by infectious bacteria. So, these compounds include antholysate, niso-lysate, collo-lysate and staphyl-lysate. Also, phage-derived compounds are the most effective ones used in phage preparation [22].
3.4. Comparison of Phages Therapy and Antibiotics
Many reports point to the same antibacterial properties of phages and antibiotics [20, 22]. In addition, much evidence suggests the same of phages and antibiotics [21, 22]. However, various studies demonstrated that phages have favorable therapeutic effects more than antibiotics. Hence, phages for the preparation of medicinal compounds can be considered more effectively in the treatment of infectious diseases. In addition, phage compounds can able to limit the spread of infectious diseases in humans with lung infections by S. aureus [23, 24].
Hence, research was conducted in the clinical phase using human samples. Also, sampling was performed in two groups A and B, that were patients with infectious diseases and was a statistical population of 223 and 117, respectively. Also, antibiotic treatment and phage therapy were applied. The results were demonstrated; the use of bacteriophages more effectively could limit the spread of the infection disease among these people [24]. The results study showed that phage therapy accelerates the treatment of infectious diseases and the resistance of bacteria in this group is also reduced [23].
3.5. Control of Biofilm Formation in Bacteria with Bacteriophage
However, the main problem in treating infections caused by pathogenic bacteria is antibiotic resistance. So, the treatment of bacterial infections with specific phenotypic conditions is a major problem. Today, biofilm formation by large bacterial populations is one of the biggest challenges for the treatment community [25-28].
On the other hand, Bacterial biofilms are a complex community of bacteria enclosed in a glycocalyx coating that adheres to the mucosal surfaces. Also, the development of biofilms can cause bacterial resistance to antimicrobial agents. In addition, biofilms in hospitals cause contamination and transmission of infectious diseases. In addition, Bacterial biofilm causes 80% of microbial infections. So, antibiotic treatment and eradication of bacterial biofilm are extremely difficult. In addition, biofilm plays an important role in nosocomial infections and urinary tract infections that can cause chronic infections. Also, chronic infection due to biofilm formation can make a huge burden in health care systems [26, 29].
Bacteriophage, as a natural and non-toxic antimicrobial compound, has found a special place in various industries. One of the new uses of bacteriophages is against biofilm-producing bacteria. In recent years, studies were performed to replace chemicals with bacteriophages to remove biofilm-producing agents from surfaces [27]. According to the evidence, various studies on the use of bacteriophages in the removal and control of biofilms of pathogens such as Klebsiella pneumoniae, Listeria monocytogenes, Pseudomonas aeruginosa and S. aureus in culture medium have been successful [30]. Also, the effectiveness of bacteriophages on biofilm is considered to be influenced by bacteriophage characteristics such as biofilm formation environment and type of bacteria and bacterial characteristics [31] (Fig. 2).
3.6. Modify Host Attachment Properties
Evidence demonstrated that by genetically manipulating the bacteriophage, the binding way the bacteriophage to the host can be changed. So, the host of the M13 filament phage is E.coli. Also, phage M13 is altered by special techniques to be able to bind to H. pylori. Also, various fragments of an antigen-binding chain were amplified in mice and produced monoclonal antibodies against H. pylori. On the other hand, these fragments were expressed as g3p binding protein on the surface of phage M13. So, this recombinant phage was able to kill different strains of H. pylori. This technology will be used in places where a high virulence lytic phage cannot be found for a specific bacterium [31, 32].
3.7. Phage Therapy and Bioterrorism
Today, the challenge of bioterrorism is becoming one of the most controversial issues in the entire world [32]. In addition, bioterrorism is the use of biological weapons to carry out destructive acts against humanity including in various forms, including the use of transgenic animals, genetically engineered bacteria, contaminated food, and dangerous toxins. So, weapons of mass destruction, including nuclear weapons, biological agents, chemical agents, incendiary weapons or other unconventional weapons, have the potential to threaten health, safety, food and the environment. So, in biological warfare, microorganisms and their associated toxins are used to produce disease and death in humans. Also, bioterrorism is a terrorist activity in which biological agents are used to assassinate individuals [33].
Unlike conventional weapons, biological agents can destroy agricultural products, cause food poisoning and contamination of medicinal products. However, because phages effectively kill pathogenic bacteria, these microorganisms can be used to counter bioterrorism threats. Although there is still no tool in society to deal with the threat of bioterrorism, it seems that due to the high potential of phages to eliminate bacterial contamination, these microorganisms can soon help treat infectious diseases [32, 33].
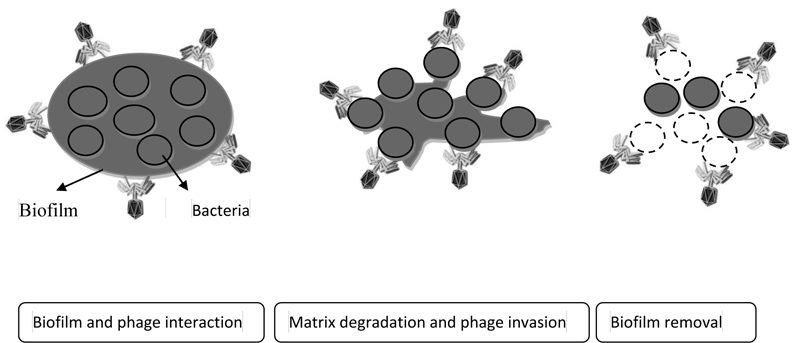 |
Fig (2). Schematic figure of phage therapy against bacterial biofilm formation [25-27]. |
4. LIMITATIONS
In the present review study, one of the problems was the lack of access to articles in this field. Therefore, we tried to increase the comprehensiveness of the search as much as possible by using the right keywords and the combination of keywords. On the other hand, research deficiencies in previous studies are other limitations of this study. Hence, the need for further developments in this field of study is suggested.
CONCLUSION
In summary, bacteriophages can be used effectively in the treatment of bacterial infections. In addition, phages are due to their many properties in killing different types of bacteria. They can be used as new therapeutic targets in the development of new drugs in the treatment of bacterial infections. However, in various studies, the use of phages as new drugs in limiting the growth of infectious bacteria is of high priority and importance. Therefore, it seems that more research needs to be done in this area. In this regard, it is necessary to use more advanced techniques to evaluate and design new phages. On the other hand, several systematic studies on the safety and toxicity of phage therapy in animals and humans have been reported in various journals. In general, in studies, after summarizing safety and toxicity data in phage therapy, potential approaches to optimize safety and toxicity monitoring using phage therapy are being developed. In many studies, phage therapy-related side effects were observed, but these side effects were rare and required further study. We suggest that more extensive studies be done on this issue [34].
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The current study was supported by Hamadan University of Medical Sciences, Hamadan, Iran.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the valuable comments and suggestions of the Dr.Kourosh Sayehmiri, Dr. Behzad Pourhossein and Dr. Maryam Fazeli which have improved the quality of this paper.







