All published articles of this journal are available on ScienceDirect.
Effects of Coronary Angioplasty on Inflammatory Markers and Lipid Profile in Patients with Cardiovascular Disease
Abstract
Background:
Although atherosclerosis is a major cause of mortality, little is known about the role of inflammation, and mediators of disease progression. In this study, serum levels of inflammatory markers were evaluated in stable atherosclerotic disease patients before and by 24 hours after coronary angioplasty and stenting.
Methods:
The study included 12 patients (eight women and four men) who underwent coronary angioplasty to implant a conventional wire-mesh cobalt-chromium stent. Changes in the lipid profile were investigated. The pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukine-17 (IL-17) were measured by enzyme immunoassays. All patients received statins and reported being hypercholesterolemic, hypertensive, and sedentary.
Results:
TNF-α and IL-17 levels did not differ significantly before and after angioplasty. The total leukocyte count had a significant reduction when compared before (7.6; 6.5-10.6cells/µL) and after (6.78;5.2-8.2cells/µL) angioplasty, although, on the other hand, the CRP levels increased from 2.5 (0.0-14.75) to 8.0 (0.75-31mg/dL) (p<0.05). Patients had significantly higher average total cholesterol before (160; 148-193) then after (155; 122-172mg/dL) (p=0.0038), as well as HDL-cholesterol, before (41; 30-49) and after (33; 32-42mg/dL) (p=0.0192), and apolipoprotein-A levels, before (159;133-169) and after, (143; 115-150 mg/dL) (p<0.05) procedure. On the other hand, no significant differences were noticed on LDL-cholesterol, triglycerides, and apolipoprotein-B concentrations.
Conclusion:
The angioplasty procedure with stent implantation influenced lipoprotein metabolism specifically that of HDL, by leading to HDL-c and apolipoprotein-A reductions, as well as total leukocyte count, and CRP elevations by 24 hours after procedure.
1. INTRODUCTION
Cardiovascular Diseases (CVDs), including coronary heart diseases, are associated with multiple risk factors, including smoking, diabetes, obesity, hypercholesterolemia, sedentarism, and a family history of CDV [1]. Equal numbers of men and women are affected, but the risk of CVD increases in women after menopause [2].
The main CVD is atherosclerosis, which arises from inflammation and endothelial dysfunction. In the course of atherosclerosis, the vascular endothelium disrupts vascular homeostasis and provokes adaptive and functional alterations by the release of several pro- and anti-coagulant substances. Atherosclerosis is a worldwide public health issue and represents the principal cause of morbimortality in the Occident [3].
Various plasmatic biomarkers are related to the atherosclerotic inflammatory process, including C-Reactive Protein (CRP), a pro-inflammatory marker associated with vascular dysfunction and atherosclerotic progression. High sensitive CRP (hsCRP) is a strong predictive risk factor for cardiovascular events or death and can lead to alterations in the pro-atherosclerotic profile [4, 5]. In acute inflammatory conditions, the hsCRP concentration increases during the first 6-8 hours and can rise to 300mg/dL in 48h [6]. Serum hsCRP values less than 1 mg/dL are related to a low risk of CVD development, while 1-3mg/dL hsCRP indicates an intermediate risk, and 3 mg/dL CRP indicates a high risk [7].
Macrophages are the primary source of cytokines in atherosclerotic plaques [8]. Many pro-atherogenic cytokines are involved in autoimmune diseases, including rheumatoid arthritis (e.g. IL-6, IL-17, and TNF-α) [9]. TNF-α is a pleiotropic cytokine with a large inflammatory effect on atherosclerosis; it is produced by several cell types, including lymphocytes [10].
IL-17 stimulates pro-inflammatory factors involved in host defence against bacterial infections and contributes to atheroma plaque stability [11]. It is produced by T CD4+and Th17 cell lineages [12]. IL-17 drives endothelial cells and macrophages to secrete several inflammatory mediators (e.g. IL-6 and TNF-α) and metalloproteases (MMPs) [13].
The total leukocyte count is another indicator of inflammatory status [14]. Despite the low cost and wide availability of this analysis, its predictive value has not been explored extensively [15].
Dyslipidemia has essential roles in the physiopathology of Coronary Artery Disease (CAD) and, consequently, in atherosclerosis. The cardiovascular risk progressively increases as the concentrations of Low-density Lipoprotein cholesterol (LDL-c) and Apolipoprotein-B (apo-B) increase, and declines as the concentrations of High-Density Lipoprotein cholesterol (HDL-c) and apolipoprotein-A (apo-A) increase [16].
In advanced stenosis cases or when there is a vascular occlusion in CAD, coronary angioplasty is the most common method for myocardial revascularization, and coronary stent implantation (expansible wire graft) is the standard procedure; it is the safest approach and yields optimal results compared to those of balloon catheterisation [17].
The atherosclerotic physiopathogenic process involves oxidation and inflammation. Thus, in this study, the expression levels of inflammatory markers, lipid profile, apolipoprotein-A (apo-A), and apolipoprotein-B (apo-B) were evaluated in CAD patients before and by 24 hours after coronary angioplasty and stent placement, as well as the significance of these biomarkers changes on the expandable manipulation of the atherosclerotic plaque.
2. MATERIALS AND METHODS
2.1. Study Design
A cross-sectional study with a convenience population sample consisted of 12 stable CAD patients of both sex, aged over 42 years, with an angiographically documented, isolated single-anterior descending coronary artery obstruction, luminal diameter stenosis ≥70% (usually estimated by 2 experienced vascular surgeons) in the proximal or midvessel location were candidates for the study. All patients were recruited from Jun 2013 to December 2013, at the Cardiology Section-hemodynamic Unit of Ana Nery Hospital, which belongs to Professor Edgar Santos Hospital Center from the Universidade Federal da Bahia (UFBA), Salvador, Bahia, Brazil. The behaviour of cardiovascular risk biomarkers was assessed before and by 24 hours after a Percutaneous Coronary Intervention (PCI) balloon angioplasty with a conventional stent (PRO-Kinect Energy Cobalt Chromium (CoCr) Coronary Stent System, small and medium stent design - Biotronik®). The PCI procedure followed a standard protocol, accomplished with a small balloon catheter inserted into the wrist or groin arteries, according to medical criteria, advancing to the narrowing in the coronary artery. The selected patients did not use previous high doses statins, the majority of them were using simvastatin or atorvastatin regularly as shown in Table 1. They were maintained in a PCI standard protocol (i.e. 75mg Clopidogrel alone or plus 100mg aspirin, both once a day), as well as taking regular medication before and after PCI according to the individual medical condition (i.e. antihypertensive therapy). Individuals with hepatic or renal dysfunction, and/or thyroid dysfunction were not included in the study (Flow Chart).
2.2. Ethics Statement
This research complied with all relevant national regulations and institutional policies, and was in accordance with the doctrine of the Helsinki declaration. Participants were previously advised and signed an informed and free consent agreement. The ethical terms of the research were approved by the Ethics Committee of the Maternidade Climério de Oliveira from the UFBA, nº 029/2014.
2.3. Laboratory Analyses
Blood samples were collected by venipuncture from the stable patients (immediately before PCI procedure and by 24 hours) to evaluate inflammatory markers associated with cardiovascular risk. To determine lipid and cholesterol concentrations, fresh serum was used. To the measurement of IL-17 and TNF-α cytokine levels, and hsCRP, an acute phase protein concentrations, serum was used on average after monthly storage at -70°C. To obtain leukocyte differential counts, an EDTA blood sample was used at the PCI procedure day.
| – | Female | Male | Total |
|---|---|---|---|
| – | (n=8) | (n=4) | (n=12) |
| Age (years) | 55 (47-60) | 56 (48-61) | 56 (48-61) |
| Tabagism | 0 | 1 | 1 |
| Hypertension | 8 | 4 | 12 |
| Diabetes Mellitus | 5 | 1 | 6 |
| Alcoholism | 0 | 0 | 0 |
| Sedentarism | 8 | 4 | 12 |
| Family history of dyslipidemia | 3 | 1 | 4 |
| Atorvastatin (40mg)a | 3 | 0 | 3 |
| Simvastatin (20mg, 40mg)a | 5 | 4 | 9 |
Values are presented as absolute numbers, except for age that was shown as median and interquartile range.
After the angioplasty procedure, patients were kept for 24h in the hospital intensive therapy unity, according to the directives of the Brazilian Society of Cardiology [18]. As lipid profiles exhibit intense changes after 24h, due to the inflammatory response, consequently, blood was collected by 24h after coronary angioplasty.
The capture immunoenzymatic assay (ELISA) was used to determine the cytokine levels in serum (i.e. IL-17 and TNF-α) using commercial kits (eBioscience, San Diego, CA, USA). To establish their concentrations, the serum samples were tested twice according to the kit instructions. The serum cytokine concentrations were expressed as pg/mL, and they were determined by using standard curves. The curves were prepared by means of consecutive known standard solutions for TNF-α (4 pg/mL to 500 pg/mL) and IL-17 (4 pg/mL to 500 pg/mL) determinations.
The hsCRP serum levels were determined by immunonephelometry using IMMAGE immune-analytical equipment (Beckman Coulter, Brea, CA, USA), following international recommendations to assess coronary risk. The employed baseline value was less than 3 mg/L.
The Total Cholesterol (TC), HDL-c, and triglyceride concentrations were obtained using the specific reagents in an automatic biochemistry analyser LABMAX 240 (Labtest Diagnóstica S.A., Vista Alegre, Brazil) with commercial sets (Labtest) following enzymatic methods. To determine the TC and TG levels, the colorimetric Trinder enzymatic method was employed. The LDL-c concentrations were estimated by Friedewald’s formula for patients with TG values up to 400mg/dL. To evaluate the lipid profile, reference values from the Brazilian population were used (i.e. CT < 200 mg/dL, LDL-c< 130 mg/dL, HDL-c> 40mg/dL, and TG <150 mg/dL) [19]. Furthermore, the apo-A and apo-B serum lipoprotein levels were measured before and by 24 hours after angioplasty using immunoturbidimetry, with baseline values of apo-A, 105-175 mg/dL, for men and 105-205 mg/dL, for women, and of apo-B, 60-140 mg/dL, for men and 55-130 mg/dL, for women.
2.4. Statistical Analysis
Descriptive and inferential statistical analyses for continuous variables were performed using the D’Agostino & Pearson test. For parametric tests, the paired t-test was used, and for non-parametric tests, Wilcoxon’s test was used. Based on the experiment design, in which parameter values were obtained before and after angioplasty, the control patients were the same patients and paired groups were evaluated. The results were considered significant when p<0.05 to a 95% confidence interval. The 25 and 75 interquartile ranges were obtained. The statistical analyses were implemented in Prism 5.1 (GraphPad Software Inc., La Jolla, CA, USA).
3. RESULTS
3.1. Socio Demographic Parameters and Medication
The population sample consisting of 12 stable CAD patients (eight women and four men, average age 56 (48-61) years (Table 1), submitted to elective coronary angioplasty for the implantation of a conventional wire-mesh Cobalt Chromium stent. Ten patients had a suggestive pain history of a previous small myocardial infarction (i.e. with the minimal time of one year, and without previous PCI or revascularization interventions). Based on the clinical history, patients had various cardiovascular risk factors, including hypercholesterolemia, hypertension, sedentarism, and tabagism. All patients used statins on a regular basis; 3/12 used atorvastatin (40 mg) and 9/12 used simvastatin (20mg or 40mg) (Table 1).
3.2. Analysis of hsCRP, Cytokines, and Leukocytes
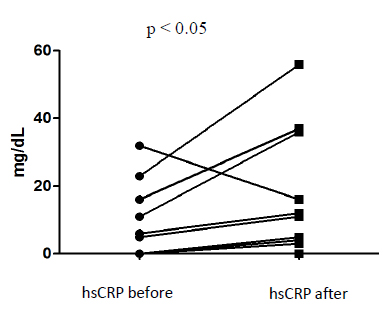
The hsCRP concentrations showed significant differences before and after angioplasty, and specifically was lower before, 2.5 (0.0-14.75) mg/dL, than by 24h after the procedure, 8.0 (0.75-31.00) mg/dL (paired t-test; p<0.05) (Fig. 1).
The cytokine concentrations (TNF-α and IL-17) did not show any significant differences before and after angioplasty (Table 2).
A global analysis of leukocytes revealed significant differences. The average leukocyte counts were greater before, 7.6 (6.5-10.6 cells/µL), than after, 6.78 (5.2-8.2 cells/µL) angioplasty (paired t-test; p < 0.05) (Fig. 2).
| Cytokine | Before | After | p-value |
|---|---|---|---|
| TNF-α | 4.18 (2.32-6.87) | 5.47 (4.07-7.44) | p > 0.05 |
| IL-17 | 1.07 (0.76-2.01) | 1.33 (0.81-1.82) | p > 0.05 |
| Biomarkers | Before | After | p-value |
|---|---|---|---|
| TC | 160 (148-193) | 155 (122-172) | 0.0038 |
| HDL-c | 41 (30-49) | 33 (32-42) | 0.0192 |
| LDL-c | 109 (90-153) | 125 (85-158) | NS |
| TG | 120 (94-223) | 110 (96-179) | NS |
| apo-A | 159 (133-169) | 143 (115-150) | <0.05 |
| apo-B | 98 (91-114) | 97 (81-116) | NS |

3.3. Lipid Profile Characterization
Before angioplasty and stenting, the patients exhibited higher average concentrations of TC, HDL-c, and apo-A (p<0.05) than those recorded after the procedure. On the other hand, LDL-c, TG, and apo-B levels did not show significant differences (p>0.05; Table 3).
4. DISCUSSION
The average age of individuals in this study and risk factors were comparable to those of most studies of CVDs [20, 21]. More women were affected; however, women typically use healthcare services more frequently than men. Since cardiovascular events impact women and men at the same frequency, the healthcare industry and politics in Brazil should emphasize the necessity to change the male perception of healthcare [22].
Both cytokines studied, TNF-α and IL-17, did not show any significant differences, but the CRP concentration (an atherosclerotic independent risk marker) and leukocyte count differed significantly before and after coronary angioplasty. In another study, the leukocyte count and CRP levels in patients with stable angina were both significantly elevated 48h after angioplasty [23]. In our study, there was a reduction in the leukocyte count 24h after the procedure. The leukocyte count before angioplasty has prognostic importance [24]. A higher leukocyte count in advance of the procedure indicates a higher disease severity, which may suggest acute myocardial infarction.
In another study [25], although they have found interleukin increase following angioplasty, the concentrations returned to basal levels after 12h. In terms of the TNF-α concentration, there were no significant alterations. In another scenario, evaluating angioplasty with stent implantation on hemodialyzed patients observed a decrease in hsCRP serum concentration only after a 9-month procedure with a pharmacologic (Paclitaxel-eluting) stent implantation, a situation that did not occur with the comparable sirolimus-eluting stent, in which the hsCRP is still elevated [26]. Other authors have shown that serum CRP increases during the first 6 to 8h after an acute inflammatory condition, as a marker of inflammation and atheroma plaque instability (atherosclerotic lesion) and can reach peak levels at 48h [6]. So all these studies corroborate our data [6, 25, 26].
A previous study [27] has shown that after angioplasty the CRP levels increased in CAD patients, and the average values were higher in individuals who were submitted to intervention with a stent than those that did not receive it. So as we observed, the CRP levels are enhanced after angioplasty in CAD patients and this may be explained by a direct mechanical effect of stent application at the atheroma plaque [28]. Therefore, accordingly, to our data, the CRP elevation suggests a strong impact of stent implantation on the coronary atheroma and its association with the simultaneous production of some inflammatory biomarkers [27].
Other studies indicate that angioplasty alone or with stent implantation, results in a systemic inflammatory response, triggering a serum CRP elevation [25]. These findings corroborate our results, which show increased serum concentrations of CRP after coronary angioplasty. This may be explained by the fact that the angioplasty physical procedure, and as well as a consequence of the restenosis, may promote the regulation of cellular migration, proliferation, and differentiation, thereby inducing a mediated acute like inflammatory reaction [29].
High-pressure balloon insufflation can cause severe barotrauma and, consequently, can exacerbate inflammation in patients with stent implantation than in those treated with angioplasty alone [30]. In spite of that, some authors showed pieces of evidences for the proatherogenic role of IL-17, via proinflammatory changes at multiple levels in atherosclerosis based animal models and also in clinical settings in cardiovascular diseases, with perspectives for IL-17-targeted cytokine therapy [31, 32]. It must be emphasized that no study has examined the role of IL-17 until now, before and after the angioplasty procedure with the implantation of a non-pharmacologic wire-mesh stent.
In this study, reductions in TC and HDL-c were detected after coronary angioplasty with stent implantation. Although the reduction of TC seems to be good, the HDL-c reduction is an already known risk factor for CVD [33]; many clinical and epidemiological studies have shown the association of a low HDL-c plasmatic concentration with the CVD development [34, 35]. Several transfer proteins participate in lipoprotein metabolism and in the regulation of HDL particle remodeling [e.g. Cholesteryl Ester Transfer Protein (CETP), Lecithin-Cholesterol Acyltransferase (LCAT), and phospholipid transfer protein (PLTP)] which facilitate lipid transport and lipoprotein cholesterol flux [33]. It is important to emphasize the decrease in apo-A values together HDL-c after stent grafting, which appears to show an HDL lipoprotein particle functional impairment (i.e. structure and/or metabolism) by the alteration of LCAT, CETP function and/or apo-A biosynthesis [33, 34]. Although the reduction of HDL-c and apo-A is an already known mechanism associated to infection and/or inflammation, in which HDL is enriched with Serum Amyloid A (SAA) protein, and on the other hand, HDL cholesterol and apo-A reduce; apo-A also behaves as a negative acute-phase reactant [36, 37]. No reports have characterized the lipid profile before and by 24h after the angioplasty intervention, which makes it difficult to better understand the data based on other studies comparison.
4.1. Study Limitations
The results of this study need to be read considering some limitations, because: a) the cross-sectional analysis does not allow to prove causality; b) the small and convenience sample does not allow generalization of our results; c) the impossibility to washout the maintenance and regular drugs administration, and finally, d) the balloon angioplasty processes with a common wire-mesh stents implantation in the atherosclerotic plaque expandable manipulation, and its implications on serum lipoprotein metabolism and other biomarkers concentrations, needs to be better addressed, and followed in prospective studies to reach sounder conclusions.
CONCLUSION
In conclusion, angioplasty with wire-mesh non-pharmacologic stent implantation acutely influenced lipoprotein metabolism specifically that of HDL, an important antiatherogenic lipoprotein particle involved in remodeling and reverse cholesterol transport, by leading to HDL-c and apo-A reductions, and CRP elevation by 24 hours after the procedure. It is important to emphasize the contribution of this study to the knowledge of coronary angioplasty plasma lipoproteins and biomarkers alteration.
LIST OF ABBREVIATIONS
| CAD | = Carotid Artery Disease |
| CRP | = C-Reactive Protein |
| ELISA | = Enzymatic Linked Immunosorbent Assay |
| TNF | = Tumor Necrosis Factor |
| IL | = Interleukin |
| HDL | = High-Density Lipoprotein |
| LDL | = Low-Density Lipoprotein |
| TG | = Triglycerides |
| TC | = Total Cholesterol |
| AMI | = Acute Myocardial Infarction |
| HDL-c | = HDL-Cholesterol |
| LDL-c | = LDL-Cholesterol |
| Apo-A | = Apolipoprotein-A |
| Apo-B | = Apolipoprotein-B |
| CCL | = Chemokine CC-motif Ligand Beta |
| CXCL | = Chemokine CXC-motif Ligand Alfa |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Federal University of Bahia´s human research ethics committee Maternidade Climério de Oliveira, Brazil (CEP/MCO/UFBA protocol number 029/2014).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all the participants.
FUNDING
Financial and infrastructural support for this study was provided by UFBA; the study was sponsored by CNPq, FAPESB, and CAPES- finance code 001.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We thank the SIDI and Clinical Biochemistry Laboratory staff from the Department of Clinical and Toxicological Analysis, Faculty of Pharmacy of the Federal University of Bahia.


