All published articles of this journal are available on ScienceDirect.
Serum Vascular Endothelial Growth Factor in Patients with Hepatocellular Carcinoma and its Validity as a Tumor Biomarker
Abstract
Background:
Hepatocellular Carcinoma (HCC) is one of the most common cancers associated with deaths worldwide and the presence of valid biomarkers for early diagnosis in high-risk patients can ameliorate the outcome of HCC. Vascular Endothelial Growth Factor (VEGF) has been found to play an essential role in the process of HCC growth and progression.
Objectives:
Therefore, we evaluated the serum VEGF levels in patients with HCC and liver cirrhosis and estimated its significant value for differentiating HCC patients from liver cirrhosis patients.
Material and methods:
Eighty-one subjects were enrolled in the study, 30 patients had HCC, 31 patients had liver cirrhosis and 20 were healthy control subjects. VEGF and AFP were measured using ELIZA. Abdominal ultrasound and triphasic abdominal computed tomography were performed in all subjects. Receiver Operating Characteristics curve analysis was performed for serum VEGF to determine its validity as a tumor biomarker.
Results:
The median levels of the serum VEGF were highly expressed in the HCC group (418 pg/ml) and the liver cirrhosis group (308 pg/ml) with no significant difference (P = 0.767); however both groups showed a significant increase compared to the control group (0.8 pg/ml, P <0.000). Serum VEGF showed high sensitivity (100%) and high specificity (100%) in differentiating HCC patients from controls with a cut-off value of ≥ 64.2 pg/ml, although it showed low sensitivity (29.2%) and specificity (85.7%) for differentiating HCC patients from liver cirrhosis patients.
Conclusion:
VEGF can be used as a reliable biomarker for differentiating HCC patients from healthy subjects but it can't be used as a reliable biomarker for differentiating HCC patients from high-risk patients as liver cirrhosis. The elevated serum VEGF levels in HCC and liver cirrhosis patients can elucidate the crucial role of angiogenesis in HCC and liver cirrhosis.
1. INTRODUCTION AND BACKGROUND
Hepatocellular Carcinoma (HCC) is the main cause of primary liver cancer [1] and the fifth common cause of cancers worldwide [2] and the second main cause of cancer-associated deaths with increasing prevalence [3]. Population based-studies showed that most patients suffering from HCC died of this disease [2] where patients who develop symptoms of HCC have survival rate between 0%-10%; [4] but with early diagnosis, the 5-year survival rate is greater than 50% [5]. The relationship between HCC and cirrhosis has been documented suggesting that Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) are the most prevalent causes of HCC [6, 7]. HCC is usually diagnosed at intermediate and late stages due to its long incubation period and insidious onset [8]. Therefore early diagnosis in high-risk population as in chronic hepatitis-related liver cirrhosis by a reliable biomarker can improve the outcome of HCC [9]. AFP is still used for diagnosis and screening of HCC [10], yet it has low sensitivity as in early stages and with small tumors [11] or has high false-positive results as with chronic hepatitis and liver cirrhosis [12]. Measurement of AFP with other liver biomarkers can increase its sensitivity and specificity [13, 14].
Angiogenesis is a dynamic process of hypoxia and growth factors where it leads to the formation of new vessels. Liver angiogenesis is either physiological as in liver regeneration or pathological as in chronic liver diseases, HCC, and metastatic liver cancer [15]. Vascular Endothelial Growth Factor (VEGF) is the main leading force for the physiological and pathological angiogenesis and is found to be highly expressed in HCC [16]. VEGF enhances tumor angiogenesis by various means as accelerating the endothelial cells migration, invasion, survival enhancing the vascular permeability through attachment to specific endothelial cells receptors [17]. Various studies assessed the crucial role of VEGF in the development, growth, metastasis, angiogenesis in HCC patients [18, 19]. Also, researchers demonstrated the crucial role of angiogenesis in the process of liver fibrosis [20, 21]. Therefore we assessed the serum levels of VEGF in HCC patients and patients with HCV related liver cirrhosis and evaluated its validity as a reliable biomarker for differentiating HCC patients from liver cirrhosis patients.
2. MATERIAL AND METHODS
2.1. Subjects
We performed this case-control study from April 2017 to June 2018 on 81 subjects divided into 3 groups. Group (1) included 30 patients having HCC, group (2) included 31 patients having Liver Cirrhosis (LC) secondary to HCV infection (HCV related LC) from preliminary 80 liver cirrhosis patients selected from the inpatients of Kasr Al Ainy University Hospital, Internal Medicine Department and group (3) included 20 healthy age-matched control subjects.
2.1.1. Inclusion and Exclusion Criteria
In our study, we included adult patients known to have HCC diagnosed clinically and radiologically by triphasic abdominal CT as recommended by the European Association for the Study of the Liver (EASL) guidelines [22] and patients known to have liver cirrhosis due to HCV irrespective of age and sex. We excluded patients with liver cirrhosis secondary to HBV infection, autoimmune, metabolic liver diseases and on hepatotoxic drugs. Fig. (1) shows a flow chart for the inclusion and exclusion criteria of the study.
2.1.2. Ethical Aspects
Research protocol was approved by the medical ethics committee of Kasr Al Ainy Hospital Medical School, Faculty of Medicine, Cairo University. All the participants provided informed consent for the research protocol. The research protocol follows the ethical guidelines of the 1975 declaration of Helsinki.
2.1.3. Laboratory Tests
Full history and clinical examination were performed in all subjects. Only serum blood sample was taken for measurement of complete blood picture, Alanine Transaminase (ALT), Aspartate Transaminase (AST), Gamma-Glutamyl Transferase (GGT), Prothrombin Time (PT), Prothrombin Concentration (PC), International Normalized Ratio (INR), urea, creatinine, hepatitis B surface antigen, hepatitis C virus antibody, AFP and VEGF. Abdominal ultrasound and triphasic abdominal Computed Tomography (CT) were performed in the studied groups. Serum VEGF level was measured by the human Quantikine® ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
The Quantikine® Human VEGF Immunoassay is a 4.5 hour solid-phase ELISA ready to calculate human VEGF165 levels in serum. It includes Sf 21-expressed recombinant human VEGF165 and antibodies raised against the recombinant protein. Results gained for naturally occurring human VEGF and recombinant human VEGF121 exhibited linear curves that were, as the standard curves, obtained using the Quantikine® kit standards. These conclusions indicate that we can use this kit to set the relative mass values for natural human VEGF.
2.2. Principle of the Method
The human VEGF kit is a solid phase sandwich Enzyme Linked-Immuno-Sorbent Assay (ELISA). The samples together with the standards, controls and unknowns were poured in the wells of microtiter strips together with a polyclonal human VEGF antibody and left to incubate where the human VEGF bound to the immobilized antibody after washing and biotinylated monoclonal antibody specific for human VEGF was added and left for second incubation where the biotinylated monoclonal antibody bound with the already captured immobilized antibody in the first incubation. The second wash was given again to remove the excess antibody formed, then Streptavidin-Peroxidase (enzyme) was added which bound to the biotinylated antibody to form the four-member sandwich. After a third incubation and wash to remove the entire unbound enzyme, a substrate was added where color was generated and its strength was observed to be positively correlated to the human VEGF concentrates present in the original sample. When color generation stopped we calculated its strength.
2.2.1. Statistical Analysis
Data was analyzed through the Statistical Package of Social Science Software program, version 23 (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.). Data were presented using the median and inter-quartile range for quantitative variables and frequency and percentage for qualitative ones. Normality was checked through the Kolmogorov-Smirnov test to choose the right inferential statistical test. A comparison between groups for quantitative variables was carried out using Kruskal Wallis tests followed by the Mann Whitney test for pair-wise comparisons while the comparison of qualitative variables was performed through Chi-square test. P values less than 0.05 were considered statistically significant. Figures (Box plots) were used to illustrate some information. Receiver Operating Characteristics (ROC) curve analysis was carried out for VEGF and AFP. Youden's J statistic was calculated for each cut-off point as follows [sensitivity + specificity -1], the selected cut-off point was the one that achieved the higher Youden's J value considering the higher sensitivity and specificity.
3. RESULTS
Our HCC group included 25 (83.3%) men and 5 (16.7%) women with mean age (58.6±8.1 years). The HCV LC group included 16 (51.6%) men and 15 (48.4%) women with mean age (54.3±6.3 years), and the control group included 10 (50%) men and 10 (50%) women with mean age (48.9±12.9 years). History of gastrointestinal bleeding was found in 21 (67.7%) HCC patients and 22 (73.3%) HCV related LC patients. By examination, we detected jaundice in 12 (40%) HCC patients and in 7 (22.6%) HCV related LC patients, lower limb edema in 15 (50%) HCC patients and in 19 (61.3%) HCV related LC patients and hepatic encephalopathy in 17 (56.7%) HCC patients and in 16 (51.6%) HCV related LC patients. By abdominal ultrasound, we detected ascites in 22 (73.3%) patients in the HCC group and in 21 (67.7%) patients in the HCV LC group. We also found portal vein thrombosis in 10 (33.3%) patients in the HCC group and in 12 (38.7%) patients in the HCV liver cirrhosis group. By computed tomography, intraabdominal lymphadenopathy was detected in 5 (16.7%) patients in the HCC group and also single hepatic focal lesion was detected in 9 patients, 2 lesions in 6 patients, 3 lesions in 3 patients and 4 lesions in 3 patients.
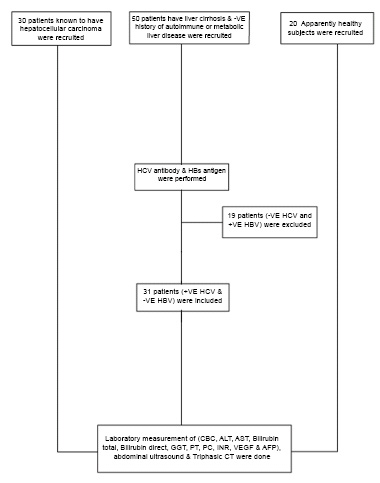
Table 1 shows the demographic and laboratory data of the studied groups. There was a statistically significant difference in age (P = 0.006) with significant (male) predominance in the HCC group (P = 0.014) (Table 1).
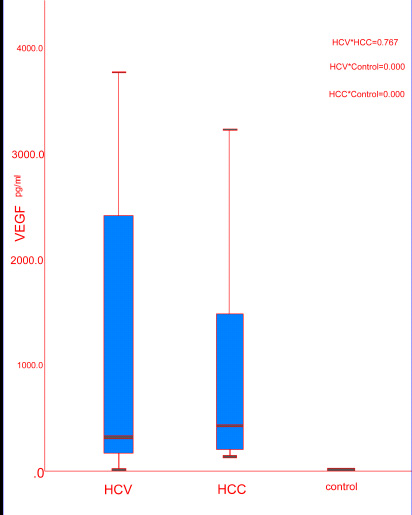
In our study, the serum levels of VEGF were significantly different by comparing the three groups (P < 0.000). By comparing serum VEGF levels between every 2 groups, we found no significant difference in the median serum VEGF levels in HCC group (418 pg/ml) compared to HCV LC group with median (308 pg/ml, P = 0.767) but there was a significant increase in their median serum VEGF levels compared to the control group (0.8 pg/ml, P < 0.000) (Table 2, Fig. 2). For serum AFP, there were no significant differences in the median serum levels of AFP in patients with HCC (9.4 ng/ml) compared to patients with HCV LC (9.8 ng/ml, P =0.609) and control group (27 ng/ml, P = 0.057) (Table 2).
To detect the relationship between serum VEGF expression and portal vein thrombosis, we found a significant increase in the median serum levels of VEGF (1686 pg/ml) in patients having portal vein thrombosis in the HCC group (10 patients) compared to HCC patients (20 patients) who did not have portal vein thrombosis (242 pg/ml, P = 0.008) but this comparison was not significant in the HCV LC group (Table 3).
| - |
HCC (n=30) |
HCV (n=31) |
Control (n=20) |
P value |
|---|---|---|---|---|
| Age (years) | 58.6 ± 8.1 | 54.3 ± 6.3 | 48.9 ± 12.9 | 0.006* |
| Sex | - | - | - | - |
| Male | 25 (83.3%) | 16 (51.6%) | 10 (50%) | 0.014* |
| Female | 5 (16.7%) | 15 (48.4%) | 10 (50%) | |
| Hb (gm/dl) | 9.7±1.9 | 9.4±2.3 | 12.2±1.4 | 0.000* |
| TLC mm3 | 10.4±5.9 | 8.9±6.4 | 7.2±2.3 | 0.247 |
| PLTs µL | 142.8±82.6 | 110.1±57.8 | 261±78.8 | 0.000* |
| ALT (U/L) | 110.2±187.7 | 58.5±29.3 | 45.7±7.1 | 0.051 |
| AST (U/L) | 246±47.2 | 82.7±39.6 | 60.93±13.83 | 0.012* |
| Albumin (gm/dl) | 2.14±0.58 | 2.26±0.48 | 3.66±0.31 | <0.001* |
| Bil Total (mg/dl) | 3.4±3.5 | 3.7±5.3 | 0.8±0.2 | 0.004* |
| Bil Direct (mg/dl) | 2.33±2.74 | 1.74±2.60 | 0.41±0.19 | 0.003* |
| PT (sec) | 18.26±3.76 | 18.79±3.90 | 12.26±0.29 | <0.001* |
| PC % | 50.42±14.37 | 52.13±13.41 | 99.24±1.03 | <0.001* |
| INR | 1.80±0.71 | 1.67±0.44 | 1.04±0.11 | <0.001* |
| - | HCC (1) (n=30) | HCV (2) (n=31) | Control (3) (n=20) | P value | 1*2 | 2*3 | 1*3 |
| VEGF (pg/ml)Range | 3420 – 127 | 0.8-3760 | 0.1 - 1.3 | ||||
| Median (IQR) | 418 (198-1480) | 308 (160.5 - 2416) | 0.8 (0.4 - 1) | 0.000* | 0.767 | 0.000* | 0.000* |
| AFP (ng/ml)Range | 0.6 – 953 | 1.2 – 311 | 14- 45 | ||||
| Median (IQR) | 9.4(2.8 – 26.2) | 9.8 (2.7 – 255) | 27 (21 – 33) | 0.165 | 0.609 | 0.484 | 0.057 |
| - | Portal Vein Thrombosis in HCV LC Group | P value | |
|---|---|---|---|
| - | Yes (12) | No (19) | - |
| VEGF (pg/ml) | - | - | - |
| Range | 0.8 - 3112 | 93 - 3760 | - |
| Mean ± SD | 1654 ± 1385.4 | 726.1 ± 1010 | - |
| Median (IQR) | 2416 (131 - 2821) | 304 (222 - 720) | 0.482 |
| - | Portal Vein Thrombosis in HCC Group | P value | |
| - | Yes (10) | No (20) | - |
| VEGF (pg/ml) | - | - | - |
| Range | 221 - 3420 | 127 - 2100 | - |
| Mean ± SD | 1834.1 ± 1322.1 | 536.2 ± 604.4 | - |
| Median (IQR) | 1686 (664 - 3166) | 242 (184 - 712) | 0.008* |
To detect the relationship between VEGF with other parameters, we found that the serum levels of VEGF and AFP were strongly correlated significantly both in the HCC group (r = 0.868, P = 0.000) and HCV LC group (r = 0.685, P = 0.000). There was no significant correlation between the VEGF and the number of hepatic focal lesions (r = 1.09, P = 0.637). Moreover, there were no significant correlations between VEGF and (age, Haemglobin, PLTs, WBCs, albumin, AST, direct bilirubin, INR) in the HCC group or HCV LC group (Table 4).
By using ROC curve analysis, we found that the cut-off value ≥ 64.2 pg/ml of serum VEGF showed 100% sensitivity and 100% specificity with AUC = 1.00 to differentiate HCC patients from control group but we found low sensitivity (23.8%) and high specificity (100%) for AFP to differentiate HCC patients from control group (Table 5, Fig. 3).
Also, we found that the cut-off value ≥ 47.2 pg/ml yielded 91.7% sensitivity and 100% specificity with AUC = 0.968 for serum VEGF to differentiate HCV LC patients from the control group. In addition, low sensitivity 41.7% and specificity 100% for serum AFP were found in differentiating HCV LC patients from control with a cut off value of ≥ 47.5 ng/ml (Table 6, Fig. 4)
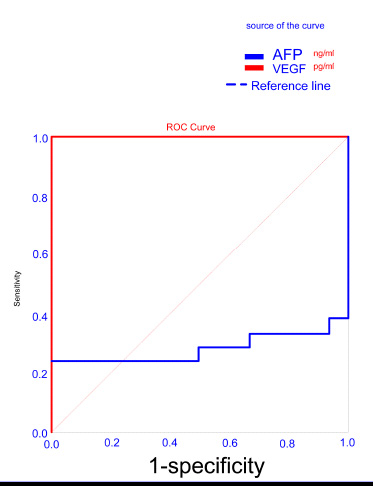
Due to the nonsignificant difference in serum VEGF levels between HCC patients and HCV LC patients, we found low sensitivity of 29.2% and specificity of 85.7% with a cut-off value ≥ 2281 pg/ml for VEGF to differentiate HCC patients from HCV LC patients and we found low sensitivity for AFP for differentiating HCC patients from patients with liver cirrhosis (41.7%) with AUC of 0.545 and specificity of 76.2% with a cut-off value ≥ 38.1 ng/ml (Table 7, Fig. (5).
4. DISCUSSION
Most studies reported an increased expression of serum VEGF levels in patients with HCC and its reliability for diagnosis of HCC in high-risk patients with liver cirrhosis together with AFP [16, 23, 24]. The increased expression of VEGF in HCC patients was due to the increased tissue transcript of the VEGF level in the tumor [25], where VEGF enhanced the angiogenesis and increased the vascular endothelial cells, leading to cancer formation [26]. Therefore, this study evaluated its value in patients with HCC and liver cirrhosis and further analysed its significance in differentiating patients with HCC from high-risk patients as HCV related liver cirrhosis. Interestingly, we found contradictory results. We did not find a significant difference in the median serum levels of VEGF between HCC patients and HCV LC patients but we found significant elevations in their median serum VEGF levels in comparison to the control group and these findings are in agreement just with two studies [27, 28].
Giatromanolaki et al., also reported significantly increased levels of VEGF in patients with cirrhosis than control and they postulated a crucial role of VEGF in hepatic fibrosis progress through increased fibroblast stimulation [29]. Raluca et al., reported the high expression of VEGF in the liver tissue of chronic hepatitis and liver cirrhosis patients, and they assumed that this elevation is related to malignant transformation of liver cells. They further suggested that early use of anti-VEGF treatments might decrease the risk of neoplastic transformation [30]. Interestingly, Deli et al., assessed VEGF in the hepatic tissue and serum in patients with liver cirrhosis and HCC and found a significant elevation in VEGF in the surrounding liver cirrhosis tissue than the HCC tissue which correlated with the serum VEGF levels [31].


| - | HCV Group | HCC Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | VEGF | AFP | VEGF | AFP | ||||||||
| - | r | P | r | P | r | P | r | P | ||||
| AFP | 0.685 | 0.000* | - | - | 0.868 | 0.000* | - | - | ||||
| Age | -0.076 | 0.724 | -0.194 | 0.365 | -0.098 | 0.674 | -0.042 | 0.858 | ||||
| Hb | -0.113 | 0.599 | -0.146 | 0.496 | 0.120 | 0.605 | 0.279 | 0.221 | ||||
| TLC | -0.204 | 0.338 | 0.120 | 0.575 | 0.225 | 0.328 | 0.333 | 0.140 | ||||
| PLTs | -0.285 | 0.177 | -0.333 | 0.112 | 0.105 | 0.650 | 0.208 | 0.366 | ||||
| ALT | 0.064 | 0.768 | -0.033 | 0.880 | 0.232 | 0.312 | 0.448 | 0.042* | ||||
| AST | 0.118 | 0.583 | -0.192 | 0.369 | 0.233 | 0.309 | 0.324 | 0.152 | ||||
| Alb | -0.201 | 0.346 | 0.111 | 0.607 | -0.080 | 0.729 | -0.162 | 0.484 | ||||
| Bil T | -0.096 | 0.656 | 0.044 | 0.840 | 0.128 | 0.581 | 0.166 | 0.472 | ||||
| Bil D | -0.107 | 0.619 | 0.166 | 0.437 | 0.271 | 0.235 | 0.330 | 0.144 | ||||
| PT | 0.040 | 0.854 | 0.407 | 0.048* | 0.271 | 0.234 | 0.197 | 0.393 | ||||
| PC | -0.502 | 0.013* | -0.282 | 0.181 | -0.192 | 0.405 | -0.122 | 0.598 | ||||
| INR | 0.232 | 0.276 | 0.327 | 0.119 | 0.149 | 0.520 | 0.146 | 0.527 | ||||
| - | AUC | 95% CI | Cut-off Value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| VEGF | 1.00 | 1.000-1.000 | ≥64.2 | 100% | 100% |
| AFP | 0.280 | 0.099-0.462 | ≥171.5 | 23.8% | 100% |
| - | AUC | 95% CI | Cut-off Value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| VEGF | 0.968 | 0.918-1.000 | ≥47.2 | 91.7% | 100% |
| AFP | 0.436 | 0.242-0.631 | ≥47.5 | 41.7% | 100% |
| - | AUC | 95% CI | Cut-off | Sensitivity | Specificity |
|---|---|---|---|---|---|
| VEGF | 0.474 | 0.303-0.646 | ≥2281 | 29.2% | 85.7% |
| AFP | 0.545 | 0.372-0.717 | ≥38.1 | 41.7% | 76.2% |
Hepatic fibrosis progression to liver cirrhosis and associated complications as HCC is closely related to pathologic angiogenesis [15], where hypoxia is a common finding from early injury until the development of liver cirrhosis. The regeneration nodules, fibrous septa and vascular remodeling with sinusoidal capillarization all lead to decreased oxygen diffusion [32], which increases the VEGF transcription and protein synthesis in the liver cirrhosis [33]. This can explain the higher serum expression of VEGF and the crucial role of angiogenesis in liver cirrhosis and HCC.
AFP can enhance tumor cell proliferation, invasion and metastasis by stimulating apoptosis of immune cell, binding AFP receptors and inducing signal transduction pathway [8]. In our study, we did not find a significant difference in the serum AFP levels in the HCC group compared to the HCV cirrhosis group or control group as we found that some of our HCC patients had low serum AFP levels and the HCV cirrhosis group had high serum AFP levels. Researchers explained the low levels of AFP in the HCC group that not all HCC tumors can secrete AFP in the serum [34, 35]. In a large Turkish study, the serum AFP was assessed in 1773 HCC patients and 57.9% of patients were found to have low serum AFP ranging from 20-100 IU/ml, mostly detected in patients with small-sized less aggressive tumors but low levels were also detected in some large-sized tumors [36]. The elevated levels of serum AFP in our cirrhotic group were also found in the study by Tai et al., who observed elevated AFP levels in cirrhosis without HCC [37]. The elevated levels were found in patients with hepatitis and liver cirrhosis most properly due to increased hepatocyte turnover present with hepatic injury, inflammation or necrosis [38, 39].
HCC patients who have portal vein thrombosis are usually associated with poor prognosis [40]. Also, patients with cirrhosis have a high incidence of portal vein thrombosis, especially patients with child B and C [41]. In our study, the serum levels of VEGF were highly expressed in HCC patients with portal vein thrombosis, which was not observed in patients with liver cirrhosis. Similarly, other studies found a significant increase in serum VEGF in patients with HCC with portal vein thrombosis compared to patients without this complication [42, 43]. Li et al., postulated that VEGF can enhance portal vein thrombosis formation in HCC by influencing the angiogenesis process [43]. On the other hand, we did not find a significant correlation between the serum VEGF levels and the number of hepatic focal lesions in contrast to other researchers [24, 44] where VEGF accelerates vascular permeability which increases the release of malignant cells into the circulation [45].
In our study, we found that serum VEGF levels strongly correlated with serum AFP which agrees with other studies [24, 46, 47], and this supports the prognostic value of both VEGF and AFP in HCC patients [47]. According to the relationship between VEGF and liver function tests, we did not find significant correlations between them in both the HCC group and HCV liver cirrhosis group which agrees with the results obtained by Atta et al. [24], where they postulated that VEGF expression cannot be considered as an indicator of the liver synthetic function or the inflammatory state which eliminates the possibility that inflammatory process has an impact on VEGF expression. On the contrary, Jaroszewicz et al., found positive correlations between serum VEGF and liver enzymes and hypothesized that serum VEGF expression can show the degree of hepatic function impairment in liver cirrhosis [48].
In our study, we found high sensitivity (100%) and high specificity (100%) for the serum VEGF in differentiating HCC patients from controls with a cut-off value of ≥64.2 pg/ml, while we found low sensitivity (23.8%) and high specificity (100%) for AFP to detect HCC patients from controls. In the study by Yvamoto et al., serum VEGF was found to have 40% sensitivity and 96% specificity with AUC= 0.709 for detecting HCC patients from control and AFP showed 28% sensitivity and 99% specificity with a cut-off value of 200 ng/ml to detect HCC patients from controls [23]. Therefore, AFP is not a reliable method for screening HCC because of its low sensitivity [5]. This can elucidate the use of serum VEGF as a noninvasive serological marker for diagnosing HCC from healthy subjects.
Interestingly, we also found that serum VEGF showed high sensitivity (91.7%) and high specificity (100%) in detecting HCV LC patients from controls with a cut-off value of ≥47.2 pg/ml. We also found low sensitivity and specificity to diagnose HCC from HCV LC patients, thus elucidating the crucial role of VEGF in the process of angiogenesis in both disorders and its unreliability to be used as a tumor marker for diagnosing HCC in high-risk patients of liver cirrhosis.
There were some limitations in our study: the relatively small sample size of our studied groups, patients were not in the early disease stages and it is preferable to measure these biomarkers in early stages of cirrhosis and HCC to detect their validity for early diagnosis.
CONCLUSION
Serum VEGF levels were highly expressed in both HCC and HCV LC patients which elucidates the crucial role of angiogenesis in both HCC and liver cirrhosis. Also, serum VEGF levels were highly expressed in HCC patients with portal vein thrombosis which expresses their value in prognosis. Serum VEGF level showed high sensitivity and specificity in detecting HCC patients from controls so it can be used as a reliable serological marker for diagnosing HCC patients from healthy subjects, but it cannot be used as a marker for diagnosing HCC patients from high-risk patients as HCV liver cirrhosis. Further studies are still needed to detect the role of VEGF in different chronic liver diseases and other reliable biomarkers for early detection of HCC from high-risk patients.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research protocols were approved by the medical ethics committee of Kasr Al Ainy Medical School, Cairo University.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All participants provided informed consent after the research protocols were carefully explained to them.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None
CONFLICT OF INTERESTS
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
None


