All published articles of this journal are available on ScienceDirect.
The Teratogenic Mechanism of Echinochrome as a Hypoglycemic Agent on Wistar Rats
Abstract
Background:
Echinochrome (Ech) is the active ingredient in the Histochrome drug, which possesses strong antioxidant, hypolipidemic and hypoglycemic activity.
Objective:
The present work aimed to characterize the malformations induced by moderate and high dose of Ech during pregnancy.
Methods:
In this study, eighteen (18) female pregnant rats were assigned into 3 groups (6 rats/ group); control group, low dose Ech (0.1 mg/kg) and high dose Ech (1 mg/kg).
Results:
The high dose of Ech caused a significant decrease in the number of embryos, uteri weight, body weight gain, placenta weight, and embryo weight and length. Also, the high dose led to a significant increase in serum AST, ALT, ALP, urea and uric acid of mothers.
Conclusion:
Our findings revealed the first teratogenic effects of high dose Ech. The teratogenic mechanism of Ech works through induction of the hypoglycemic condition in pregnant rats.
1. INTRODUCTION
In developed countries, the use of prescription drugs is common during pregnancy with a prevalence ranging from 27 to 99%, depending on the data sources used and medication included [1]. Current evidence suggests that between 65%-94% of women take at least one prescription drug during pregnancy [2]. Some chemical agents and drugs can induce teratogenic effects and abortion anomalies result from the disruptive [3]. It is estimated that 7 to 10% of human anatomic actions of drugs, viruses, and other environmental factors [4]. A substantial number of medical drugs are suspected to induce birth defects through various mechanisms, including folate antagonism, vascular disruption and oxidative stress [5].
Among marine bioactive compounds, Echinochrome (Ech) is a natural quinone pigment isolated from sea urchin shells and spines that possess many biological activities [6]. Ech is the active substance in the drug Histochrome which is widely used in Russia. Histochrome drug is used in ophthalmic practice to treat intraocular hemorrhage, diabetic retinopathy, dystrophies, central retinal vein thrombosis, and post-traumatic hemorrhage in Russia [7]. Some authors suggested that the phenolic hydroxyl groups in these molecules could take part in antioxidant activity, as was observed in other well-known antioxidant polyphenols such as the catechins [8]. In addition, recent studies used Ech in the treatment of diabetes mellitus in rats [9]. Ech is a powerful antioxidant which acts through many antioxidant mechanisms, including the scavenging of active oxygen radicals [10], interaction with lipoperoxide radicals [11], chelation of metal ions [12], inhibition of lipid peroxidation [12], and regulation of the cell redox potential [7]. Thus, Ech will be involved in the treatment of many diseases, but till now, human teratogenic risks of Ech are unknown. Thus, the current study considers the first experiment on Ech as an example of hypoglycemic drugs on pregnancy and discusses the mechanisms involved.
2. EXPERIMENTAL
2.1. Chemicals and Reagents
Dimethyl Sulfoxide (DMSO) and Echinochrome were purchased from Sigma-Aldrich (St. Louis, MO, USA). The biochemical analysis kits were purchased from the Biodiagnistic Company (El Moror St, Dokki, EGY).
2.2. Sample Collection
During November 2017, sea urchins (Paracentrotus lividus) were collected from the Mediterranean coast of Alexandria, Egypt. After collection, the samples were carefully cleaned with seawater to remove sand and overgrowing organisms and then transferred to the laboratory packed in the icebox.
2.3. Extraction of Echinochrome (Ech)
Ech pigment was extracted according to the method adopted by Amarowicz et al. [13] with some modifications. The shells and spines were cleaned with a stream of cold water after the removal of internal organs. Then, the samples were air-dried at 4°C for 2 days in the dark. Dried samples were hand crushed and then coarsely powdered with a grinder. Thereafter, five grams of dried sample was gradually hydrolyzed with 10 mL of 6 M HCl. After hydrolysis, Ech pigment was extracted 3 times with 10 ml of diethyl ether. The collected ether layer was washed by adding NaCl (5%) until the acid almost removed. Finally, the ether solution containing pigments dried over anhydrous sodium sulfate and the solvent was evaporated under reduced pressure. Ech pigment was stored at -30°C in the dark until use [14].
2.4. Experimental Animals
Twenty-seven Wistar rats (18 virgin female, 7-8 weeks old, 200-220 g; and 9 mature male, 14-15 weeks old, 240-260 g) obtained from the National Research Center (NRC, Dokki, Giza) were transferred to our breeding colony and maintained on a 12 h light/dark cycle and controlled temperature (23 ± 2 oC). The animals were given food and water ad libitum.
2.5. Ethical Consideration and Field Study
Experimental protocols and procedures used in this study were approved by Cairo University, Faculty of Science, Institutional Animal Care and Use Committee (IACUC) (Egypt) (CUIF718). All the experimental procedures were carried out under international guidelines for the care and use of laboratory animals.
2.6. Reproductive Management
After one week of acclimatization, mating between males and females (1: 2) was done. The presence of sperm in the vaginal lavage will be considered day 0 of gestation.
2.7. Experimental Design
Eighteen (18) pregnant rats were divided into 3 groups (6 rats/ group).
- Group 1 (control): Rats were given orally 1 mL 2% Dimethyl Sulfoxide (DMSO) daily during pregnancy period.
- Group 2: Rats were given a low dose of Ech orally (1 mL of 0.1 mg) daily during pregnancy period.
- Group 3: Rats were given a high dose of Echinochrome orally (1 mL of 1 mg/kg) [15] daily during pregnancy period.
At day 20, the animals were euthanized and the blood samples were collected for biochemical analysis. For embryo: morphological malformation was observed.
2.8. Biochemical Analyses
The serum glucose, serum aspartate Aminotransferase (AST) and Alanine aminotransferase (ALT), Alkaline Phosphatase (ALP), total bilirubin, creatinine, urea uric acid, Total Cholesterol (TC), Low-Density Lipoprotein (LDL-C) and High-Density Lipoprotein (HDL-C) were determined according to the manufacturer’s instructions using Spectrum Diagnostics and Bio-diagnostic kits (Giza, Egypt).
2.9. Statistical Analysis
Values were expressed as means ±SE. The comparisons within the groups were made using one-way Analysis of Variance (ANOVA) with Duncan post hoc test used to compare the group means. P<0.05 was considered statistically significant. SPSS, for Windows (version 15.0) was used for the statistical analysis.
3. RESULTS
The effect of Ech on glucose and liver function markers is shown in Table 1. The results showed that a low dose of Ech did not cause a significant change (p> 0.05) in glucose and liver function markers. However, the high dose caused a significant increase (p<0.05) in liver function markers and a significant decrease in glucose level..
Data represented in Table 2 show that Ech caused a significant decrease (p<0.05) in TC, TG, LDL-C and a significant increase in HDL-C.
According to the data recorded in Table 3, non-significant change was noticed in kidney function makers after the administration of a low dose of Ech. While significant increases (p<0.05) were observed in the levels of urea, creatinine and uric acid at a high dose of Ech.
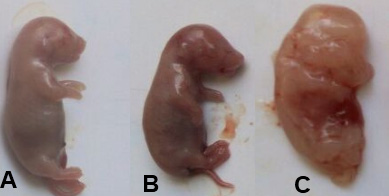
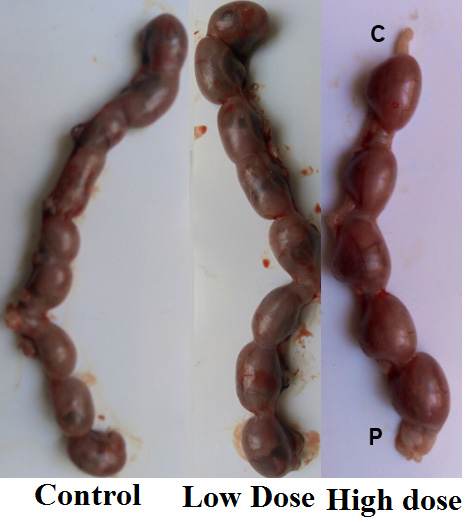
Non-significant changes (p> 0.05) in the number of fetuses, uteri weight, body weight gain, placenta weight, fetus’s weight and length of a low dose of Ech group were observed. While significant (p<0.05) reductions were noticed in a number of fetuses, uteri weight, body weight gain, placenta weight, fetus’s weight and length in a rat group treated with a high dose of Ech. (Table 4, Figs. 1 and 2).
| Groups | Glucose (mg/dL) |
AST (U/mL) |
ALT (U/mL) |
ALP (U/L) |
Bilirubin (mg/dL) |
TP (g/dL) |
Albumin (g/dL) |
| Control | 93.12±1.48 | 136.25±1.36 | 36.11 ±1.90 | 234.41±1.03 | 0.75±0.06 | 5.38±0.24 | 3.15±0.12 |
| Low dose | 85.02±3.21 | 145.00±1.7 | 40.25 ±2.54 | 232.01±2.43 | 0.68 ±0.06 | 5.81±0.45 | 3.32±0.21 |
| High dose | 77.13±2.98* | 157.20±3.84* | 50.4 0±2.51* | 244.20±2.88* | 0.65±0.02 | 7.02±0.29* | 4.08±0.13* |
| Groups | TC (mg/dL) | LDL-C (mg/dL) | HDL-C (mg/dL) | TG (mg/dL) |
| Control | 183.25±2.09 | 147.3±4.36 | 35.25±2.35 | 149.5±3.76 |
| Low dose | 173.50±2.09* | 135.11±3.44* | 45.75±3.16* | 138±3.24* |
| High dose | 160.60±2.13* | 130.53±3.64* | 50.8±3.04* | 136±2.82* |
| Groups | Urea(g/dL) | Creatinine(mg/dL) | Uric Acid(mg/dL) |
| Control | 29.25±0.66 | 0.73±0.04 | 2.75±0.51 |
| Low dose | 34.25±1.02 | 0.93±0.11 | 2.97±0.31 |
| High dose | 44.4±3.92* | 1.2±0.08* | 3.24±0.34* |
| Groups | Fetuses Number | Live Fetuses | Dead Fetuses |
Uterus Weight (gm) |
Body Weight Gain (gm) | Placenta Weight (gm) | Fetuses Weight (gm) |
Fetuses Length (cm) |
| Control | 13.21±0.37 | 13.21±0.37 | 0 | 28.88±1.30 | 37±0.95 | 0.36±0.01 | 3.88±0.05 | 5.56±0.03 |
| Low dose | 14.62±0.24 | 14.62±0.24 | 0 | 26.1±0.48 | 30.6±1.08 | 0.37±0.01 | 3.74±0.07 | 5.39±0.04 |
| High dose | 6.11±0.32* | 2.84 ±0.21* | 3.12± 0.12* | 7.52±0.56* | 21.6±0.81* | 0.27±0.01* | 1.63±0.02* | 4.21±0.08* |
4. DISCUSSION
Ech, the active ingredient in the Histochrome drug, is a water-insoluble compound that possesses strong antioxidant activity [16]. In addition, Ech is considered to be a strong hypolipidemic agent that possesses the ability to improve renal function [17, 18]. Many recent studies revealed the hypoglycemic activity of Ech [9, 17, 19-22]. Our results showed that the administration of Ech at high doses caused a significant increase in AST and ALT activities. The high dose of Ech resulted in hypoglycemic state, which is characterized by enhanced muscle catabolism [23]. The amino acids released by muscles are used by the liver for gluconeogenesis. Thus, increases in amino acids lead to an increase in the urea's activity cycle enzymes including AST and ALT. According to our results, alkaline phosphatase activity increased after the administration of a high dose of Ech. A recent study revealed that Ech increases insulin production [9]. In response, as the insulin secretion increases, the alkaline phosphatase activity increases [24]. The increase in serum albumin and total protein levels after the administration of a high dose of Ech may be related to extreme muscle protein breakdown during hypoglycemia [23].
The treatment with Ech showed decreases in the concentration of TG, TC, LDL-C and increased level of HDL-C, which may be due to an increase in insulin level [25].
The high dose of Ech caused an increase in urea concentration, which is related to the activation of the urea cycle to consume increase in amino acids produced from protein catabolism [26]. Our results showed that the high dose of Ech increases the level of creatinine. This is due to the activation of creatinine kinase enzyme during hypoglycemia which is primarily because of damage in muscle [27].
Our data showed the teratogenic effect of the high dose of Ech. The effect was confirmed by the reduction of the fetus’s weight and length, uteri weight, body weight gain and placenta weight. The high dose of Ech caused a hypoglycemic condition in healthy pregnant rats. The maternal hypoglycaemia during early pregnancy induces teratogenic effects on various systems [28]. During the early phase of organogenesis, the embryo depends on uninterrupted glycolysis and the inhibition of glycolysis (glycolytic flux) results in dysmorphogenesis [29]. Also, growth retardation and gross development anomalies in rat embryos are subject to brief maternal hypoglycaemia [30]. In addition, prolonged maternal hypoglycaemia leads to pathological changes in the central nervous system of fetuses and neonates [31].
High blood urea as in the present experiment is thought to affect embryos by decreasing pH in the uterine lumen [32]. Decreasing media pH during incubation of fertilized zygotes was found to inhibit progression to blastocyst [32]. The previous study reported that elevated creatinine level leads to decreased fetal survival rate [33].
CONCLUSION
Our findings revealed the first teratogenic effects of a high dose of Ech. The teratogenic mechanism of Ech works through the induction of the hypoglycemic condition in pregnant rats.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The experimental protocols and procedures used in this study were approved by Cairo University, Faculty of Science, Institutional Animal Care and Use Committee (IACUC) Egypt (CUIF718).
HUMAN AND ANIMAL RIGHTS
No humans were used in this research.The reported experiments were in accordance with the standards set forth in the 8th Edition of Guide for the Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw /Guide-for-thecare-and-use-of-laboratory-animals.pdf) published by the National Academy of Sciences, The National Academics Press, Washington DC, United States of America.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTERESTS
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


