All published articles of this journal are available on ScienceDirect.
Evaluation of Fucosylated Haptoglobin as a Diagnostic Biomarker for Hepatocellular Carcinoma in Egypt
Abstract
Background:
Most Hepatocellular Carcinomas (HCCs) are diagnosed at an advanced stage. Therefore, there is citation-type an urgent need for better methods of detection and surveillance of patients at risk of HCC. Alpha-fetoprotein (AFP) has a suboptimal diagnostic performance for HCC surveillance, so novel and reliable diagnostic biomarkers are required.
Objective:
The aim of this work is to evaluate fucosylated haptoglobin as a diagnostic biomarker for hepatocellular carcinoma in Egyptian patients.
Materials and Methods:
This case-control study was carried out on 60 patients classified into 3 groups (20 patients on each); group I (HCC group), group II (Cirrhotic group) and group III (Control group). Diagnosis of liver cirrhosis was done by clinical, biochemical and ultrasound (US), whereas the diagnosis of HCC was done by percutaneous biopsy or radiological (by US and triphasic Computerized Tomography (CT) based on the guidelines of the American-Association for the Study of Liver Diseases. HCC stage was clinically defined according to the Barcelona Clinic Liver Cancer (BCLC) staging system. AFP & fucosylated haptoglobin levels were estimated in all groups.
Results:
There was a statistically significant positive correlation between serum fucosylated haptoglobin and tumor size in the HCC group. Serum fucosylated haptoglobin (at 116 U/ ml) showed sensitivity 80%, specificity 65%, positive predictive value 53% and negative predictive value 87% with AUC 0.786. Combination of serum fucosylated haptoglobin and serum AFP at (200 ng/ ml) increased sensitivity that reached 95%.
Conclusion:
Serum fucosylated haptoglobin may serve as a novel diagnostic biomarker for the detection of HCC with higher sensitivity than AFP.
1. INTRODUCTION
Hepatocellular Carcinoma (HCC) is common worldwide and is the fifth most common cancer in the world and the third most common cause of cancer-related deaths [1, 2]. In Egypt, HCC is the fourth most common type of cancer and the second leading cause of cancer death in both sexes [3-7].
Because of the high rate of morbidity, highly aggressive course, high frequency of recurrence after intervention and resistance to traditional treatments, the 5-year survival rate of patients with untreated hepatocellular carcinoma is <5%, being among worst-predicted cancer [8, 9]. However, the prediction can be improved by early diagnosis, optimal treatment and early detection of recurrence. When diagnosed at an early stage, treatment can be curative. In fact, most HCCs are diagnosed at an advanced stage. Therefore, there is an urgent need for better methods to assess and monitor people at risk of hepatocellular carcinoma [10, 11].
Alpha-fetoprotein (AFP) is the most vital biomarker for the diagnosis of HCC. However, AFP has a sub-optimal diagnostic performance for HCC surveillance. Firstly, there is also increase in AFP levels in patients with chronic hepatitis and cirrhosis [11, 12]. Secondly, only a small proportion of HCC at an early stage (10-20%) present with elevated AFP level [13], so new and reliable diagnostic biomarkers are required to complement the AFP [14].
Habtoglobin (Hp) is one of the glycoproteins secreted by the liver mainly and can be used as a new non-invasive and potential biomarker in multiple types of solid tumors such as HCC, pancreatic cancer, stomach cancer and colorectal cancer. The main function of Hp is to bind and carry free hemoglobin for degradation in the liver and for iron recycling. It captures hemoglobin released from erythrocytes and restrains the oxidative activity of hemoglobin to prevent the kidney from damage when intravascular hemolysis occurs. Hp also has catalytic, antioxidant and antibacterial capacity in regulating the acute-phase response. In addition, it serves as a natural antagonist for receptor-ligand activation of the immune system and plays a role in the stimulation of angiogenesis [15]. A unique pattern of glycoform type of Hp consisting of Fucosylated Hp was found in patients with hepatocellular carcinoma. Glycosylation is involved in critical cancer cell processes, such as cell signaling, cell differentiation, invasion, ligand-receptor interactions, and metastasis formation [16]. The Hp-T3 glycopeptide (VVLHPN241YSQVDIGLIK) carried the highest variety of multiply fucosylated glycoforms that included the Ley-type tumor-associated carbohydrate antigen (260). Pompach et al. [17] observed that hyper-fucosylated glycoforms with more fucoses (up to six fucoses per glycan) were associated with the presence of Ley-type glycoforms on Hp-T3 glycopeptide in HCC patients [18]. Therefore, fucosylated hapto globin may serve as a new marker for HCC and combined measurement of fucosylated haptoglobin and AFP can be used to complement the limitations of AFP measurement [19]. The aim of this work is to evaluate fucosylated haptoglobin as a diagnostic biomarker for hepato cellular carcinoma in Egyptian patients.
2. MATERIALS AND METHODS
This case-control observational cross-section study was carried out at Tropical Medicine Department, Tanta University Hospitals from January 2018 to August 2018. A total number of 81 patients were enrolled in this study; of these 60 patients were classified into three groups; group I (HCC group): 20 patients with HCC, group II (Cirrhotic group): 20 patients with the cirrhotic liver disease without HCC, and group III (Control group): 20 apparently healthy individuals.
The study protocol complies with the ethical guidelines of the 1975 Declaration of Helsinki. All data of the patients were confidential with secret codes and private file for each patient. After protocol approval by the ethical committee (number of approval: 31422104117), written informed consent was taken from each patient or relatives. Each patient received an explanation of the purpose of the study.
Inclusion criteria: Adult patients (18 year or more), with diagnosis of liver cirrhosis (based on clinical, biochemical and radiological criteria “by US (coarse echogenic pattern, bulky caudate lobe, attenuated hepatic veins” with or without liver biopsy) or with diagnosis of HCC was by the following criteria: pathological HCC diagnosis by percutaneous biopsy, or Clinical and radiological (by ultrasound and triphasic CT) based on the guidelines of the American Association for the Study of Liver Diseases. HCC stage was clinically defined according to the Barcelona Clinic Liver Cancer (BCLC) staging system.
Exclusion criteria: Patient refusal, patients with diabetes mellitus, chronic renal impairment, patients with extrahepatic metastases, other neoplasms, and previous history of HCC ablation and pathological or radiological evidence of mixed HCC-cholangio cellular carcinoma.
All included patients were subjected to: detailed history taking, full clinical examination, investigations: Laboratory investigations: (routine laboratory investigations: Complete Blood Count (CBC), coagulation profile: (INR, Prothrombin time and activity), kidney function tests: (Serum creatinine and urea), liver function tests: (Total and direct bilirubin, ALT, AST, serum albumin), hepatitis markers (HBs Ag and HCV antibody), fasting and postprandial blood glucose & serum level of AFP and specific laboratory investigation: serum fucosylated haptoglobin. Radiological examination: Pelviabdominal ultrasound for the presence of cirrhosis and portal hypertension and to assure or exclude the presence of focal hepatic masses and triphasic C.T. scan for suspected patients with HCC (patients with focal lesion).
Test principle of serum fucosylated haptoglobin: A double-antibody sandwich enzyme enzyme-linked immunosorbent assay (ELISA) kit was used. fucosylated haptoglobin (Fuc-Hpt) was added to monoclonal antibody enzyme well which was pre-coated with human fucosylated haptoglobin (Fuc-Hpt) monoclonal antibody, incubated, then HP antibodies labeled with biotin and combined with streptavidin-HRP to form immune complex were added. Again incubation and washing were carried out to remove the combined enzyme. Then chromoen solution A and B were added. The color of the liquid changed into blue, and by the effect of acid, the color finally became yellow. The chroma of color and the concentration of the human substance Fuc-Hpt of the sample were positively correlated.
The primary outcome of the study was the diagnostic performance of serum fucosylated haptoglobin, while secondary outcomes were level comparison and correlation with tumor size.
The sample size needed was calculated by the MedCalc program version 18.2.1 (MedCalc Software, Ostend, Belgium) using the criteria of 80% power and an alpha of 0.05 for the comparison AUC of AFP (0.7) [20], with the assumption that fucosylated haptoglobin could have an AUC (0.9). At least 18 for HCC, and 36 for control (cirrhotic and healthy) were needed and we added 6 cases more to compensate for the dropped-out cases.
All statistical analyses were performed with SPSS for Windows (version 25, SPSS Inc., Chicago, IL, USA). For quantitative data: mean and standard deviation were calculated and comparison by ANOVA (Analysis of variance) test was used with post-hoc test (Tuckey) if p-value <0.05. For qualitative data: frequency and percentage were calculated and comparison between the two groups was done using Chi-square test (X2). Pearson linear correlation coefficient (r) was used for detection of correlation between two quantitative variables. Evaluation of the diagnostic performance of each test was assessed by ROC curve analysis and the Area Under the Curve (AUC) evaluated the overall test performance. The level of significance was adopted at P-value < 0.05.
3. RESULTS
There is no statistically significant difference between the three groups regarding the age, sex and BMI (Table 1). There was a significant difference between the three groups in Hb level, platelet count, TSB, serum albumin, INR, ALT, AST but an insignificant difference between the three groups in TLC, serum creatinine and urea (Table 2).
Regarding serum fucosylated haptoglobin, there was a significant difference between the HCC group and cirrhotic group, HCC group and control group and between cirrhotic group and control group. As regard serum AFP, there was a significant difference between the HCC group and control group and between the HCC group and the cirrhotic group. However, there was insignificant difference between cirrhotic group and control group (Table 3).
There was a statistically significant positive correlation between serum fucosylated haptoglobin and tumor size in the HCC group (r=0.487, p value=0.029) (Fig 1). But there was no statistically significant positive correlation between serum AFP and tumor size in HCC (r=0.26, p value=0.263).
| HCC Group | Cirrhotic Group | Control Group | Test | P Value | |
| Age (y) | |||||
| Mean + SD | 55.4 + 7.6 | 54.25 + 8.25 | 52.9 + 9.01 | F = 0.19 | 0.827 |
| Gender | |||||
| Male | 16 (80%) | 11 (55%) | 14 (70%) | X2 =2.927 | 0.231 |
| Female | 4 (20%) | 9 (45%) | 6 (30%) | ||
| BMI (kg/m2) | |||||
| Mean + SD | 28 + 5.43 | 28.9 + 5.2 | 28.6 + 7.69 | F = 0.105 | 0.9 |
| HCC Group | Cirrhotic Group | Control Group | X2 | P Value | |
| D.M. | 5 (25%) | 1 (5%) | 1 (5%) | 5.175 | 0.075 |
| HTN | 3 (15%) | 1 (5%) | 2 (10%) | 1.111 | 0.573 |
| Bilharziasis | 4 (20%) | 4 (20%) | 1 (5%) | 2.352 | 0.308 |
| HBV | 2 (10%) | 0 | 0 | 4.137 | 0.126 |
| HCV | 9 (45%) | 20 (100%) | 0 | 40.177 | <0.001* |
| History of Hepatic encephalopathy | 4 (20%) | 0 | 0 | 8.571 | 0.013* |
| Jaundice | 3 (15%) | 0 | 0 | 6.315 | 0.042* |
| Pallor | 3 (15%) | 0 | 0 | 6.315 | 0.042* |
| Hepatomegaly | 2 (10%) | 3 (15%) | 0 | 3.054 | 0.217 |
| Splenomegaly | 9 (45%) | 0 | 0 | 21.176 | <0.001* |
| Ascites | 6 (30%) | 0 | 0 | 13.333 | 0.0013* |
| L.L. edema | 8 (40%) | 0 | 0 | 18.461 | <0.001* |
| CTP score | |||||
| A | 8 (40%) | 20 (100%) | - | - | - |
| B | 8 (40%) | 0 | |||
| C | 4 (20%) | 0 | |||
| X2 | 30 | ||||
| P value | <0.001* | ||||
| HCC Group | Cirrhotic Group | Control Group | F test | P Value | Tuckey | ||
| Hb (gm/dl) |
11.83 ± 1.28 | 12.55 ± 1.51 | 13.83 ± 0.97 | 11.63 | <0.0001*serum creatinine and urea | P1 | 0.079 |
| P2 | <0.0001* | ||||||
| P3 | 0.002* | ||||||
| Platelet (×103 /mm³) |
110.3 ± 86.22 | 132.7 ± 47.95 | 261.75 ± 79.9 | 24.88 | <0.0001* | P1 | 0.337 |
| P2 | <0.0001* | ||||||
| P3 | <0.0001* | ||||||
| TLC (×103 /mm³) |
5.42 ± 2.74 | 8.39 ± 6.19 | 7.63 ± 2.33 | 11.63 | 0.0702 | ||
| TSB (mg/dl) | 2.16 ± 1.46 | 1.02 ± 0.42 | 0.80 ± 0.17 | 13.67 | <0.0001* | P1 | <0.0001* |
| P2 | <0.0001* | ||||||
| P3 | 0.427809 | ||||||
| Albumin (gm/dl) | 3 ± 0.51 | 3.75 ± 0.45 | 4.21 ± 0.52 | 30.19 | <0.0001* | P1 | 0.00001* |
| P2 | <0.0001* | ||||||
| P3 | 0.0053 | ||||||
| INR | 1.42 ± 0.25 | 1.11 ± 0.15 | 1.14 ± 0.11 | 17.65 | <0.0001* | P1 | 0.00002* |
| P2 | 0.00008* | ||||||
| P3 | 0.657 | ||||||
| ALT (U/L) | 74.1 ± 36.84 | 69.49 ± 44.04 | 22.9 ± 9.01 | 14.25 | <0.0001* | P1 | 0.666 |
| P2 | 0.00001* | ||||||
| P3 | 0.00005* | ||||||
| AST (U/L) | 107.9 + 60.75 | 58.34 + 60.75 | 27.25 + 60.75 | 17.84 | <0.0001* | P1 | 0.001* |
| P2 | <0.0001* | ||||||
| P3 | 0.0261* | ||||||
| Creatinine (mg/dl) | 0.72 + 0.22 | 0.75 + 0.15 | 0.72 + 0.17 | 0.235 | 0.791 | ||
| Blood urea (mg/dl) | 29.35 + 5.06 | 31.45 + 3.73 | 28.05 + 4.86 | 2.8 | 0.07 | ||
| AFP (ng/ml) | 262.4 + 431 | 21.28 + 27.16 | 5.22 + 2.39 | 6.676 | 0.002* | P1 | 0.003* |
| P2 | 0.002* | ||||||
| P3 | 0.839 | ||||||
| Fucosylated haptoglobin (U/ml) | 452.6 + 521.9 | 247.4 + 201.9 | 18.9 + 3.7 | 8.4060 | 0.0004* | P1 | 0.049* |
| P2 | 0.0001* | ||||||
| P3 | 0.029* | ||||||
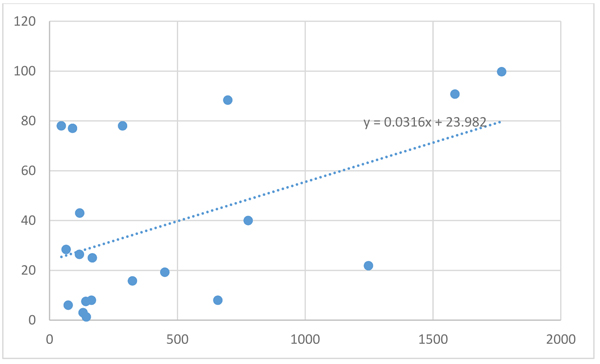
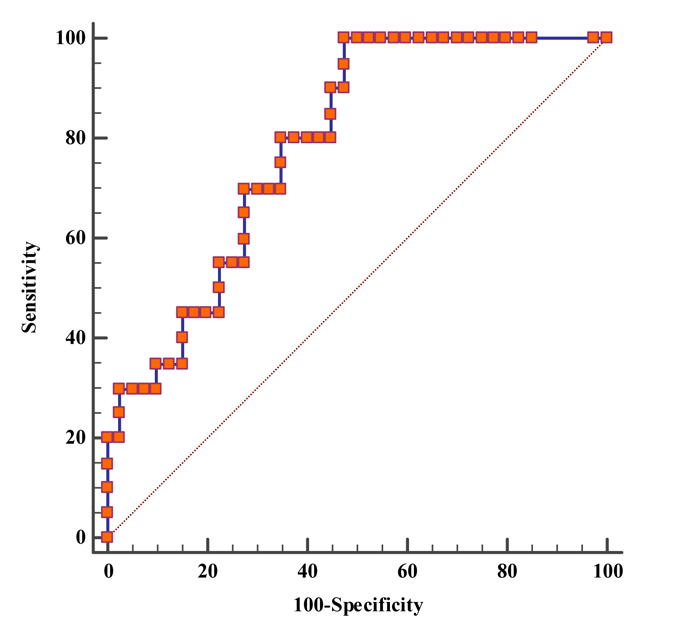
The diagnostic performance of serum fucosylated haptoglobin (with cutoff value 116 U/ml) showed sensitivity 80%, specificity 65%, positive predictive value 53% and negative predictive value 87% in the HCC group versus the cirrhotic group and the control group (Table 4). The Area Under the Curve (AUC) was 0.786 with 95% CI (0.661 - 0.882) and P-value <0.001 (Fig. 2)
Combination of serum fucosylated haptoglobin (116 U/ ml) and serum AFP (200 ng/ ml) showed a diagnostic sensitivity of 95%, specificity 65%, positive predictive value 58% and negative predictive value 96% in HCC group versus cirrhotic and control group (Table 4).
| AFP | |||||
| Cut-off | Sensitivity | Specificity | PPV | NPV | |
| 35 | 65% | 95% | 87% | 84% | |
| 97 | 55% | 98% | 92% | 81% | |
| 200 | 41% | 100% | 100% | 75% | |
| FucHapt | |||||
| Cut-off |
Sensitivity (95% CI) |
Specificity | PPV | NPV | |
| 116 | 80% (56.3 - 94.3) |
65% (48.3 - 79.4) |
53% | 87% | |
| AFP + FucHapt | |||||
| AFP | FucHapt | Sensitivity | Spec | PPV | NPV |
| 35 | 116 | 95% | 63% | 56% | 96% |
| 97 | 116 | 95% | 65% | 58% | 96% |
| 200 | 116 | 95% | 65% | 58% | 96% |
4. DISCUSSION
AFP was the only serological marker widely used to diagnose HCC. However, the sensitivity of this marker is still limited [21]. Therefore, the availability of a more sensitive serological marker that distinguishes between hepatocellular carcinoma and benign liver lesions would be very useful for the early and specific diagnosis of HCC [22].
In our study, there was a significant increase in AFP level in the HCC group than the control group (p value= 0.002) and cirrhotic group (p value= 0.003). However, there was an insignificant difference between the chronic group and the control group (p value= 0.8).
This result is in agreement with Wei et al. [22] who showed that serum AFP was significantly higher in the HCC group when compared to liver cirrhosis group or control group and referred that to the increase in selective transcriptional activation in AFP gene in the malignant hepatocytes resulting in increased secretion of AFP during the development of HCC to inhibit immune response of liver cancer cells. Zhu et al. [23] demonstrated at the cutoff value of AFP at 20 ng/ mL for HCC diagnosis a sensitivity of 51.9% with a specificity of 86.3%.
Accordingly, AFP was considered as an inadequate screening test by Sherman [24] due to the low capacity of identifying new cases not previously detected by imaging techniques. AFP levels at a value of 20 ng/ mL showed low specificity but fair sensitivity (60%); that is, AFP surveillance would miss 40%, whereas at higher cutoffs of 200 ng/ ml the sensitivity drops to 22% with high specificity. Therefore, reducing the cutoff means that more HCCs would be identified, but at the cost of a progressive increase in the false-positive rate. Sherman [25] stated that a significant limitation to the use of AFP for HCC surveillance is the rate of AFP-negative HCC. Up to 50% of small HCCs do not secrete AFP and even with larger lesions, 20% are not associated with elevated levels.
Regarding serum fucosylated haptoglobin, there was a significant difference between HCC group and chronic group, HCC group and control group and between chronic group and control group.
There was a statistically significant positive correlation between serum fucosylated haptoglobin and tumor size in the HCC group. But there was no statistically significant positive correlation between serum AFP and tumor size in the HCC group.
The diagnostic performance of serum fucosylated haptoglobin (at 116 U/ ml) showed sensitivity 80%, specificity 65%, positive predictive value 53% and negative predictive value 87% in HCC group versus cirrhotic and control group. The area under the curve (AUC) was 0.786.
Combination of serum fucosylated haptoglobin with combination of serum AFP (the cutoff value of serum fucosylated haptoglobin was 116 U/ ml and of serum AFP was 200 ng/ ml), showed a diagnostic sensitivity of 95%, specificity 65%, positive predictive value 58% and negative predictive value 96% in HCC group versus cirrhotic and control group. So, when we combine both more sensitive.
Also, the same results are found with a combination of serum Fucosylated Haptoglobin with serum AFP (the cutoff value of serum Fucosylated Haptoglobin was 116 U/ ml and of serum AFP was 97 ng/ ml).
Diagnostic performance of serum fucosylated haptoglobin with combination of serum AFP (the cutoff value of serum fucosylated haptoglobin was 116 U/ ml and of serum AFP was 35 ng/ ml), showing a diagnostic sensitivity of 95%, specificity 63%, positive predictive value 56% and negative predictive value 96% in HCC group versus cirrhotic and control group. Therefore, there was no significant difference with a combination of different cut-offs of AFP.
Our study revealed that at the cutoff 116 U/ ml, the sensitivities of fucosylated haptoglobin were significantly higher than those of AFP at cutoff 35, 97, and 200 ng/ mL (80% versus 65%, 55%, and 41% respectively), with specific ities (65% versus 95%, 98%, and 100% respectively). These indicate that fucosylated haptoglobin is a novel marker and superior to AFP with a lower false negative rate in diagnosing hepatocellular carcinomas.
Our study was similar to Shang et al. [19] who demonstrated fucosylated Hp/Hp ratio and ELISA Index, that could be potential glycobiomarkers for the surveillance and diagnosis of HCC even with a low level of AFP. Moreover, a combination of AFP and fucosylated Hp ratios could improve the sensitivity and specificity of HCC diagnosis.
The results of the current study indicated clearly that serum fucosylated haptoglobin is a promising sensitive and specific tumor marker that could be added to the current standard tests for diagnosis of HCC to detect the disease at an early stage and hence improving the prognosis and survival rate of the patient.
Further studies on early cases with a larger population including AFP-negative patients are needed to justify its implementation in clinical practice and to evaluate the significance of serum fucosylated haptoglobin.
The limitation of this study is the relatively small number of patients. Larger multi-centeric studies are needed to confirm the findings of this study. Two-thirds of HCC patients with the nodule less than 4 cm have serum AFP levels less than 200 ng/ ml and up to 20% of HCC patients do not produce AFP. It has a limited utility of differentiating HCC from benign hepatic disorders.
CONCLUSION
Serum fucosylated haptoglobin may serve as a novel diagnostic tumor marker for the detection of HCC with higher sensitivity and specificity than AFP.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the ethical committee of Tanta University Faculty of medicine (number of approval: 31422104117).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was taken from each patient or relatives. Each patient received an explanation of the purpose of the study.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTERESTS
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


