All published articles of this journal are available on ScienceDirect.
Pollution Biomarkers in Environmental and Human Biomonitoring
Abstract
Environmental pollutants generate harmful conditions for living organisms, including humans. This accounts for the growing interest to early warning tools for detection of adverse biological responses to pollutants in both humans and wildlife. Molecular and cellular biomarkers of pollution meet this requirement. A pollution biomarker is defined as an alteration in a biological response occurring at molecular, cellular or physiological levels which can be related to exposure to or toxic effects of environmental chemicals.
Pollution biomarkers have known a growing development in human and environmental biomonitoring representing a valuable tool for early pollutant exposure detection or early effect assessment (exposure/effect biomarkers).
The review discusses the recent developments in the use of pollution biomarker in human and environmental biomonitoring and analyzes future perspectives in the application of this tool such as their potentiality for bridging human and environmental issued studies.
1. INTRODUCTION
In recent years, the concentration of chemical pollutants in environmental matrices have increased dramatically as a consequence of anthropogenic activities, generating harmful conditions for living organisms, including humans.
The negative effects of pollutants are exerted at different levels of biological organization and at different time-scales. Pollutants exposure first can induce effects at the molecular, cellular and physiological levels before more integrated effects are evident at higher levels.
Loss and degradation of habitats, loss of biodiversity, and alterations of natural resources are some of the main impacts of pollution on ecosystems at higher time scales.
With respect to human health, pollution is the largest environmental cause of human disease and death in the world today, responsible for an estimated 9 million premature deaths [1], air pollution being the main environmental cause of human disease and death, followed by water pollution, occupational chemical exposure and soil pollution [1].
This growing concern towards the harmful effects of chemical pollutants on wildlife and human health accounts for the growing interest to early warning tools for the identification, estimation, and assessment of the risks posed by chemical pollutant discharges to the environment. Last years have known a developed awareness that chemical data alone of pollutant concentrations in environmental matrices (air, water, sediments, and soil) are insufficient to reliably assess the potential risks of pollution for living organisms and human health [2]. Moreover, risk assessment of chemical pollutants to organisms and ecosystems is made complex by a number of factors including: a) the diversities in chemical nature and toxic action of pollutants, b) the simultaneous presence of several pollutants in mixture that can exert additive/synergic effects on the organisms, c) the bioavailability of pollutants also influenced by a number of environmental factors, d) the different sensitivity of the organisms to pollutant exposure and effects [3].
In this complex framework, the requirement for an integrated chemical and biological approach in pollution monitoring has grown and, in turn, the interest for measurable effects of chemical pollutants on living organisms including humans has developed.
2. POLLUTION BIOMARKERS
Pollution biomarkers can be defined as quantitative measures of changes in a biological system with respect to its normal status in response to pollutant exposure. In general they are referred to changes at low levels of biological organization (e.g., molecular, cellular, physiological) [4, 5]. It is generally accepted that the effects of pollutants at lower levels occur earlier than those at higher levels (e.g., population effects). Therefore, molecular and cellular biomarkers may provide a sensitive early warning of more integrated toxicological effects that can occur later within populations [6]. In complement with the measurement of contaminants in environmental matrices, biomarkers offer a biologically relevant information on the exposure to bioavailable pollutants and on potential impacts of pollutants on the health of the exposed organisms.
Biomarkers meet the emerging need of early warning tools for detection of exposure and adverse biological responses to pollutants. This accounts for the growing development that this research field has known in recent years both in environmental sciences and human health monitoring.
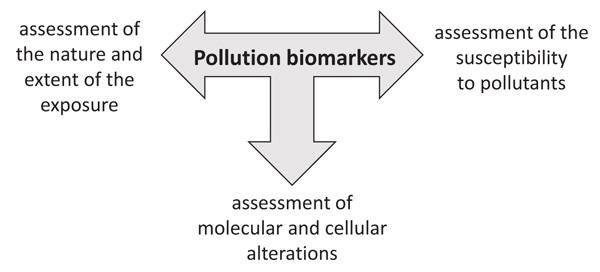
Biomarkers can be used to assess the nature and the extent of the exposure, to identify alterations occurring within an organism, and to assess underlying susceptibility of an organism (Fig. 1). They can help to increase the understanding of the processes by which a chemical is absorbed and transformed within an organism to determine alterations at the cellular and molecular levels leading to a toxic effect.
Therefore, depending on the specific biological response used as biomarker and on the point on the continuum from exposure to pathology (Fig. 2), where the measured biomarker come from, biomarkers may be classified into biomarkers of exposure, biomarkers of effect, and susceptibility [7].
Biomarkers of exposure provide an indication of the occurrence and extent of exposure of the organism to various compounds. They are early reversible cellular changes in the organism, often based on the activation of detoxification mechanisms. Biomarker of exposure can give information on the route, pathway, and, sometimes, even the source of exposure. Strictly speaking a biomarker is defined as a biological response to a chemical or a group of chemical agents. However, the measurement of a xenobiotic in a biological system or sample is often used as “biomarker of internal dose”, particularly in human biomonitoring [8]. It represents the likely concentration of a parent compound or metabolite at the target site. On the other hand “biomarkers of effective dose” are markers measured in the target tissues or surrogate tissue (such as saliva, urine) that reflect the interaction of the absorbed compound with a subcellular target. Examples of biomarkers of effective dose can be represented by alteration in enzyme activities or formation of DNA adducts or protein adducts in circulating blood cells [8].
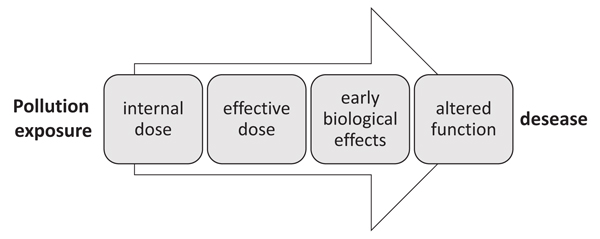
Biomarkers of effect include biochemical or physiological changes in target tissues that occur as result of exposure. They give an assessment of a toxicological effect on the organisms and are directly related to the risk of adverse health effects [9].
Biomarkers of susceptibility indicate an inherent or acquired ability of an organism to respond to specific pollutant exposure [10]. In fact, inter-individual biological differences may cause some individuals to be more susceptible to environmentally induced diseases and serve as markers of susceptibility.
The specificity of biomarkers to pollutants ranges from highly specific biomarkers such as metallothionein induction by metal (Cu, Hg, Zn, Cd) [11, 12] or aminolevulinic acid dehydratase (ALAD) inhibition by lead [13] to those that are unspecific such as DNA damage or immune system impairment. However, it has to be pointed out that when different specific biomarkers are used together, a complementation among biomarkers can be realized that results into an overall higher degree of specificity [14].
The number and type of effects that environmental pollutants, normally present in complex mixture in the environment, can exert on living organisms are very complex and multifaceted. Therefore, the use of a multibiomarker approach is strongly recommended in biomonitoring, allowing to produce results that integrate the contribution of the different routes and sources of exposure. The selection of the most relevant biomarker responses to be included in the multimarker approach in agreement with the objectives of each specific biomoni-toring program has to meet some criteria. Some of them include the sensitivity of the biomarker, its dose- and time dependent response, its biochemical memory (how long after exposure the response lasts), its natural variability [5]. In order to ensure a proper toxicity assessment, biomarkers should responds to a pollutant in a dose-dependent manner over an environmentally realistic concentration range of pollutants. Moreover, the link of the biological response used as biomarker to important biological processes and to pathological consequences is considered of relevance in both environmental assessment and heath assessment.
3. POLLUTION BIOMARKERS IN ENVIRONMENTAL BIOMONITORING
In environmental biomonitoring, measurements of biomarker responses in sensitive species (sentinel species) can be used as an early warning of alteration at population levels with the objective of monitoring environmental quality and assess changes in the environment [15].
The use of the biomarker approach in field surveys of contaminated environments has grown in the last years. This arises from the fact that biomarkers, as tool for pollutant exposure detection and effect assessment, can be useful for decision-making in a number of environmental management related activities such as ecosystem’s service and habitat protection or implementation of remediation procedures.
The EU Water Framework Directive (WFD, Directive 2000/60/EC) and the Marine Strategy Framework Directive (MSFD), that provide the guidance for monitoring programs required to assess the achievement of good chemical and ecological status of water bodies, pointed out the importance of biological monitoring for the determination of water quality [7]. In this framework specific suites of molecular and cellular biomarkers are widely used to assess impacts of environmental chemical stress on bioindicator organisms in complementation with chemical analysis on environmental matrices, which lack of information on bioavailability and toxic potential of pollutants [16, 17].
A number of biomarker responses have been studied in selected bioindicator organisms, particularly in invertebrates [18]. Some of them represent model organisms for studying effects of pollutants. Bivalve mussels and crustaceans are commonly used as sentinel organisms for biomonitoring the aquatic environment thanks to their sedentary or sessile (in the case of mussels) life, widespread distribution, and relative tolerance to pollutants. Their biochemical and cellular responses to pollutant exposure are successfully used as early warning tools in aquatic environmental monitoring and assessment [11, 19-22].
In the case of soil pollution, the measurement of biomarkers on organisms living in the soil have become of major importance for the assessment of the quality of the soil compartment [23]. Also in this case, soil invertebrates represent good sentinel organisms because they are in direct contact with soil and pore water, in contrast to many vertebrates that are indirectly exposed through the food chain [23]. Among soil invertebrates earthworms are considered relevant bioindicators of soil pollution [12, 24-29] because of their particular interactions with soil. In fact, they contribute to mineralization and humification of organic matter by food consumption, respiration, and gut passage [30].
The most assessed biomarkers in environmental biomonitoring encompass lysosomal endpoints, oxidative stress, specific responses to metals, neurotoxic pollutants and genotoxic substances.
The lysosomal system, composed by primary lysosomes, auto and heterophagic vesicles, secondary lysosomes (phagosomes) and residual corpuscles, is responsible for the breakdown of all the constituents of the cells, and endocytosed macromolecules, and it is also involved in cell defense mechanisms, in the protection against toxic agents and infections [31]. In a number of invertebrates it is known to react to pollutant exposure through alteration in lysosomal membrane stability, alteration in lysosomal number and fusion events [32-34]. These lysosomal responses, also called lysosomal activation, are related to enhanced autophagy following pollutant exposure. In particular, lysosomal membrane destabilization (assessed by lysosomal enzyme latency in frozen tissue sections or lysosomal dye retention in circulating cells) is one on the most commonly used biomarkers in invertebrates in environmental biomonitoring. It is a general biomarker of effect very sensitive to both inorganic and organic toxic chemicals in a number of sentinel species [35, 36]. The reduction of lysosomal membrane stability is often associated with an increase of the lysosome/cell volume ratio [37], which is in turn indicative of a not physiological level of cell catabolism.
The exposure to pollutants (either organic or inorganic) is known to generate oxidative stress in the cells, arising from the enhancement of reactive species and perturbation of antioxidant efficiency [38, 39]. GSH is one of the most commonly used marker of oxidative stress condition [40]. It is an important intracellular scavenger of free radicals and neutralizes peroxides in combination with glutathione peroxidase and glutathione reductase, thus maintaining the redox balance of cells. Measurement of the ratio between the reduced and oxidized glutathione (GSH/GSSG ratio) is a useful tool to assess the oxidative stress status of the organism. Lipid peroxidation products such as malondialdehyde, arising from the oxidative degradation of membrane phospholipids, represent another commonly used markers of oxidative stress. In addition, antioxidant enzymes such as catalase, superoxide dismutase and glutathione peroxidase [38, 41] have been demonstrated to be altered in their activity and expression by the exposure to either organic or inorganic pollutants, demonstrating to be general biomarkers of oxidative stress suitable for assessing effects of pollutants in aquatic and terrestrial ecosystems at early stages and with low concentrations.
The most commonly used specific response to metals used as biomarker in environmental biomonitoring is represented by metallothionein, cysteine-rich metal-binding proteins which are involved in detoxification and homeostasis of heavy metals. Their ubiquitous distribution suggests that they play a fundamental and conserved role in cells. The metal affinity for the binding site follows the general order found for inorganic thiolates, such as Hg > Cu > Cd > Zn. A number of laboratory and field studies have demonstrated their usefulness as biomarker of trace metal exposure in a number of aquatic and terrestrial bioindicator organisms [11, 12, 42].
The most assessed biomarker as response to neurotoxic compounds is represented by acetylcholinesterase inhibition. Acetylcholinesterase is a key enzyme in the nervous system, catalyzing the hydrolysis of the neurotransmitter acetylcholine. It is the target site of inhibition by organophosphate and carbamate pesticides. Monitoring of acetylcholinesterase inhibition is widely used as biomarker of organophosphorus and carbamate exposure either in aquatic [19, 43] or terrestrial environments [28]. Recently new insight are emerging in the use of acetylcholinesterase as biomarker in environmental biomonitoring. In fact, a number of contaminants other than organophosphorus and carbamate pesticides, including trace metals, detergents, hydrocarbons and some herbicides, have recently been shown to exert anticholinesterase activity [44,45]. Therefore, acetylcholinesterase inhibition appears a relevant tool for investigating biological effects of a complex mixture of many neurotoxic compounds particularly in aquatic environments.
The continuous discharge of genotoxic compounds in the environment is of major concern. Genotoxic compounds, interacting with DNA, lead to several alterations (such as point mutations, chromosomal re-arrangements, DNA adducts, DNA strand breaks and increased number of micronuclei) [46]. Considering the importance of the effects associated with DNA damage, genotoxicity biomarkers are considered of pivotal importance for identification of potential risks and adverse health effects on the biota. A large number of methods have been applied to evaluate genotoxic damage in different aquatic and terrestrial species. Comet assay, as marker for detecting DNA alterations, and micronucleus test, as marker of chromosomal damage, are the most widely applied and validated methods in field studies and frequently employed in biomonitoring programs [47].
4. POLLUTION BIOMARKERS IN HUMAN BIOMONITORING
In human biomonitoring pollution biomarkers are measured in human tissues and/or fluids from subjects currently exposed or had been exposed in the past or to be exposed to chemical risk factors in the workplace and/or in the general environment [10] with the goal to prevent the health effects of exposure to pollutants. Human biomonitoring is a useful tool for the exposure assessment of selected populations and it is currently used in surveillance programs all over the world [48].
Biomarker of internal dose are commonly measured in human biomonitoring as biomarker of exposure. Biomarkers of internal dose of compounds such as dioxins, dioxin-like PCBs and metals, which are stable in the human body, consist in the measurements of the original compound concentrations in blood, serum or urine. For chemicals that are metabolized, such as organophosphate pesticides or phthalates, metabolites of the original compound are often used as biomarkers of exposure and generally measured in urine [49]. In the last three decades several hundred exposure biomarkers have been measured in different body fluids in various populations. This amount of information has been recently collected in a database dedicated to biomarkers of exposure to environmental risk factors, the Exposome-Explorer data base (http://exposome-explorer.iarc. fr). It contains information on the nature of biomarkers, their concentrations in human specimens, the analytical techniques used for measurement, the population where they were measured, and correlations with external exposure measurements [50].
As regards effect biomarkers one of the early responses characterized in human environmental exposure and utilized in human biomonitoring is represented by the inhibition of the enzyme acetylcho-linesterase as biomarker of effect on nervous system following exposure to organophosphorus compounds [43]. Its use is increased in the last decades. Today quantification of acetylcholinesterase levels in blood is the conventional method of assessing the extent of occupational exposure to organophosphate compounds in exposed environments (for example, environments concerned with pesticide production and use). The success of this biomarker response relies in a number of characteristics necessary for the successful application of a biological response as valuable biomarker in human biomonitoring, such as its dose-dependent behavior to pollutant exposure, its sensitivity, its link to health adverse effects, and the ease with which it can be measured.
More recently, a particular emphasis in human biomonitoring is posed on the exposures to carcinogens. Therefore, the development and use of genotoxicity biomarkers is rapidly grown to measure specific occupational and environmental exposures, to predict the risk of pathological development, or to monitor the effectiveness of exposure control procedures to genotoxic chemicals [10, 49]. Micronuclei, chromosomal aberrations, 8-hydroxydeoxyguanosine (8-OHdG) and comet assay are the most commonly used genotoxicity biomarkers. In particular, assessment of DNA damage using the comet assay has been widely used in a number of human biomonitoring programmes [51, 52]. It is a sensitive and rapid technique, which can be applied to various types of cells and offer the possibility to detect various types of DNA damage, such as alkaline labile sites, single and double strand breaks, and oxidative damage [53, 54]. Micronuclei frequency in the lymphocytes and buccal mucosal cells has been commonly employed as effect biomarker to identify populations at risk, contributing to implementation of regulations and better risk management [55].
Inflammation-related biomarkers, such as cytokines /chemochines determination in human biomonitoring is an area of great promise [56]. They are considered a mainstream marker for assessing the systemic inflammatory response to external stressors [57]. Recently, inflammatory cytokines measurement in blood has been suggest as a tool for bridging alteration in physiological parameters to environmental exposures [58].
Polymorphism in genes of enzymes involved in xenobiotic metabolism are used as marker of susceptibility, because they increase the susceptibility of an individual to various xenobiotics and are associated with carcinogenesis [59]. Genotypes of polymorphisms can be detected by PCR in blood samples. For example, polymorphisms for cytochrome P450, which is a family of isozymes responsible for the biotransformation of several drugs, increased the risk of developing lung cancer and head and neck cancer several fold when present in combination with the polymorphisms for glutathione-S- transferase (GSTM1 and GSTT1) [60-62].
Another growing topic in the field of human biomonitoring is represented by oxidative stress biomarkers. Oxidative stress can be caused by many different environmental exposures and it is involved in the pathogenesis of multiple diseases. A great attention has been paid for biomarkers of oxidative damage to DNA and lipids measured in cells, tissues or biological fluids. For example oxidative stress plays a key role in the health effects of air pollution, especially particulate matter, including major outcomes such as cancer and airway and cardiovascular diseases [63]. A number of potential biomarker of oxidative stress can be suitable such as oxidation of low density lipoprotein (ox-LDL), antioxidant enzymes, and lipid peroxidation products [63, 64]. However, further studies are needed to assess the reliability and validity of oxidative stress biomarkers in human biomonitoring in relationship to a number of environmental exposures.
5. POLLUTION BIOMARKERS: TOWARDS AN INTEGRATED VIEW BETWEEN HUMAN AND ENVIRONMENTAL ISSUED STUDIES
Although a great number of works have been produced on the use of biomarkers in environmental or human biomonitoring, to date few studies have considered an integrated approach with both human and wildlife species as sentinel organisms. However, the recent growing attention to understanding the links between multiple stressors and multiple health effects has stimulated the interest for an integrated approach in biomonitoring, useful for an integrated risk assessment view.
The need of an integrated assessment of human and environmental risks has been declared by a number of international institutions and agencies, such as WHO, EPA or OECD with the aim to improve risk assessment and management and promote policy implementation [18, 65].
As suggested by Galloway et al. [66], environmental quality assessment and health risk assessment should not be considered separately, because they have strong interactions. Their integration has the potentiality to produce more realistic results and to enhance the predictive capability of the obtained data in both environmental and human health studies.
Risk assessment consists in the estimate of the probability of an adverse effect occurring as a consequence of contaminant release [67]. Human and environmental risk assessment have the main aim of the protection of humans or natural populations respectively form the harm that comes from chemical contaminants emissions. They share the same fundamental four steps procedures [68], including hazard identification, exposure assessment, dose–response assessment and risk characterization. With this regard, biomarkers can contribute to risk assessment, particularly in hazard identification, exposure assessment and to associate a response with the probability of a pathological outcome both in sentinel species and in humans. Therefore, they represent a valuable tool for developing an integrated view of risk assessment.
In many case pollutants show common molecular and cellular toxicity mechanisms of action on living organisms. With this regards, molecular and cellular biomarkers represent useful tools for bridging human and environmental issued studies (Fig. 3). For example, acetylcholinesterase inhibition is the typical mechanism of action of organophosphate and carbamate pesticides in both humans and animal organisms [44, 48]. Carbonic anhydrase inhibition have been demonstrated to be sensitive to pollutant exposure such as heavy metals and organic chemicals in both humans and a number of animal species [22, 69-73]. Stimulation of reactive species generation, alteration of the antioxidant defenses and oxidative damage to DNA and lipids are mechanisms of toxicity of several pollutants in both humans and wildlife [41, 63, 74]. Comet assay has been widely accepted as a simple, sensitive, and rapid tool for assessing DNA damage and repair in a great number of cell types in diverse species, and has increasingly found application in the biomonitoring field [75, 76].
Therefore, a number of biomarkers can be valuable for an integrated approach addressed to intervention strategies for prevention or reduction of deleterious health effects of chemical contamination in the environment as well as in humans. Recent advances in molecular biology and OMIC sciences (genomics, transcriptomics, proteomics, lipidomics, epigenomics and metabolomics, etc.) are gaining increased consideration in human and environmental biomonitoring, giving the opportunity for developing novel and more sensitive biomarkers to be utilized in an integrated approach [77-79].

Biomarkers used in an integrated biomonitoring approach can contribute to a better understanding of the exposure routes and of mechanisms underlying adverse effects in both humans and biota. Some examples are available in literature.
Markt [80] theorized a Multi-Markered Bioindication Concept which consists in a whole concept of bioindication based on the integration of human toxicology and ecotoxicology. More recently Liu et al. [81] drew a conceptual framework for integrated environmental health monitoring based on the description of the natural-eco-anthropogenic system in which human health is considered the result of the environmental state and sustainability of natural and socio-economic environment. Therefore, environmental monitoring, biomonitoring, eco-surveillance and health surveillance are seen as interconnections between the diverse components of the whole system [81].
In a study carried out in Germany, the human internal dose of Polychlorinated Biphenyls (PCBs) and Hexachlorobenzene (HCB) measured in human blood plasma were compared with data from pine shoots, egg matter of city pigeons, earthworm, and roe deer liver, providing an integrated view of the relationships between concentrations in human and biota [82].
Kier et al. [83] reviewed human and environmental genotoxicity biomonitoring studies involving exposure to glyphosate based formulations to complement an earlier review of experimental genotoxicity studies of the herbicide.
CONCLUSION
In recent years pollution biomarkers have proved their usefulness as early warning of adverse effects in both human and environmental biomonitoring. In perspective, pollution biomarkers can represent useful tools for integrating human and environmental issued studies and bridging human and environmental risk assessment. They can contribute to improve our knowledge on the link between environmental contamination and human health and ecosystem health in a more global vision, in which human health threat can be considered part of a more complex threat to the health of the whole environment.
CURRENT & FUTURE DEVELOPMENTS
The study of pollution biomarkers should be explored more extensively in the area of integrated biomonitoring and integrated risk assessment in the coming years. In perspective, this represent a fruitful research arena for developing novel approaches in biomarker implementation in environmental and human health issued studies.
AUTHORS’ CONTRIBUTION
MGL Conceived the project, analysis of published data, writing the manuscript RC, MEG collection and analysis of published data. All authors read and approved the final manuscript
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This study was supported by PRIN (Progetti di Rilevate Interesse Nazionale) project 2010–2011 prot. 2010ARBL T7_005.


