All published articles of this journal are available on ScienceDirect.
Altered Plasma Global Arginine Bioavailability Ratio in Early-stage Alzheimer’s Disease
Abstract
Background:
L-arginine is an amino acid that can be metabolized to form several bioactive molecules including Nitric Oxide (NO). In the Central Nervous System (CNS), NO regulates various and important physiological functions. However, the involvement of L-arginine metabolism –and by extension of NO- in Alzheimer’s disease (AD) pathogenesis, has been suggested.
Objective:
To determine the Global L-Arginine Bioavailability Ratio (GABR) and NO levels (as the sum of nitrates and nitrites, NOx) in the plasma of early-stage Alzheimer’s Disease (AD) patients in order to analyze if GABR can reflect an altered NO production, to confirm the importance of L-arginine metabolism in the development of the disease, and to evaluate the putative diagnostic/prognostic value of GABR.
Method:
GABR index is an indicator of the availability of L-arginine to form NO by nitric oxide synthases. It is calculated as the ratio between the levels of L-arginine and the sum of the levels of L-ornithine and L-citrulline. Plasma amino acids are measured by high-performance liquid chromatography coupled to fluorescence detection. Nitric oxide is measured in plasma as the sum of nitrates and nitrites (NOx).
Results:
No changes were found in L-arginine levels, whereas L-citrulline and L-ornithine levels were highly increased in AD patients. We also found that GABR decreased significantly by 47.8% in AD patients, whereas NOx levels increased significantly by 46.9%. Receiver Operator Characteristic (ROC) curve analysis for GABR showed a sensitivity of 78.1 and a specificity of 90.5.
Conclusion:
Low plasma GABR levels in AD patients reflect that the L-arginine-NO pathway has turned towards NO in AD, probably being related to the nitroxidative stress involved in neurodegenerative diseases. Furthermore, increased NOx could also be involved in several altered physiological functions. Therefore, GABR is proposed as a putative useful biomarker of the disease.
1. INTRODUCTION
The L-arginine is a semi-essential amino acid that can be metabolized to form several bioactive molecules [1] including Nitric Oxide (NO). Arginases are the enzymes that convert the amino acid L-arginine into urea and the amino acid L-ornithine, whereas Nitric Oxide Synthases (NOS) are the enzymes that convert L-arginine into the amino acid L-citrulline during the synthesis of NO. L-ornithine and L-citrulline are used as precursors in the synthesis of new L-arginine as enzymatic products generated from L-arginine. In addition, L-ornithine can be converted by the enzyme L-ornithine decarboxylase to produce polyamines such as putrescine, spermidine, and spermine, which are needed for proper cell growth and function [2-5]. Thus, the global balance of useful L-arginine levels can be showed by steady-state systemic L-arginine bioavailability in relation to the amount of L-arginine catabolized via arginases and NOS. Therefore, these steady-state ratios can be used to identify and quantify altered mechanisms in which L-arginine metabolism is involved. Since NO can only be formed from L-arginine, and L-citrulline and L-ornithine are the products resulting from the metabolism of L-arginine, the Global Arginine Bioavailability (GABR) index can be calculated as the ratio between the levels of L-arginine and the sum of the levels of L-ornithine and L-citrulline and is an indicator of the availability of L-arginine to form NO by NOS. This indicator has been shown to be more suitable than the levels of L-arginine [6]. NO derived from endothelial NOS (eNOS) is a key factor for the stabilization and regulation of the vascular microenvironment [7, 8], whereas neuronal NOS (nNOS)-derived NO plays an important role in synaptic plasticity and learning and memory [9-11]. In the Central Nervous System (CNS), NO regulates various and important functions such as the maintaining of cerebral blood flow, consolidation of memory processes, facilitation of long-term potentiation, maintaining of sleep-wake cycles and normal olfaction [12]. However, several authors suggest the involvement of L-arginine metabolism –and by extension, of NO- in Alzheimer’s Disease (AD) pathogenesis [12-15]. In AD brains, neurofibrillary tangles and senile plaques are associated with reduced capillary expression of eNOS [16-18]. Also, eNOS-derived NO can directly modulate the production of amyloid beta (Aß) avoiding its increase [19]. However, NO also acts as a free radical, and an excessive amount of NO, mainly derived from inducible NOS (iNOS), leads to neurotoxicity and neurodegeneration due to nitroxidative stress instead of oxidative stress [14, 20, 21]. It has been also reported that NO produced in response to Aß promotes mitochondrial fission, synaptic loss, and neuronal damage [22]. On the other hand, L-ornithine can also be used to produce glutamate that can be further metabolized to generate the amino acids Gamma-Aminobutyric Acid (GABA) and glutamine through the enzymes glutamic acid decarboxylase and glutamine synthase, respectively [1, 23, 24]. It must be also taken into account that it has been described decreased levels of glutamate and GABA in brains of AD patients and increased glutamine synthase enzyme in cerebrospinal fluid [25, 26].
We examine here the GABR in early-stage AD patients to analyze if this parameter can reflect an altered NO production, which would confirm the importance of L-arginine metabolism, and by extension, of NO, in the development of the disease, and if so, whether there is diagnostic/prognostic significance in measuring GABR in this patients. To our knowledge, it has not been described yet in the literature plasma GABR values in patients with AD but is presented as a potential early and non-invasive biomarker for the illness.
2. MATERIALS AND METHOD
2.1. Subjects
The subjects of this study were 46 individuals with AD (20 males and 26 females; age 74.82 years ± 1.10) and 46 healthy age-matched controls (16 males and 30 females; age 73.63 years ± 0.96). Patients were recruited from the Unit of Neurology of the University Hospital “Ciudad de Jaén” and healthy voluntaries from routine controls. People who take antibiotics, Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), steroids, vitamins or antioxidant supplements were excluded. Subjects with a history of smoking and alcohol intake were also excluded from the study. Subjects received a diagnosis of AD if they met DSM-IV clinical criteria for dementia, and received a diagnosis of probable or possible AD according to NINCDS/ADRDA (National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association) criteria. People with AD who had comorbidity with other clinical major neurological illness were excluded.
Cognitive and functional status of AD patients was assayed by Mini-Mental State Examination (21.89 ± 0.58); Blessed Scale (6.98 ± 0.46) and Brief Cognitive Rating Scale (BCRS) adapted as Functional Assessment Stage (FAST) (3.80 ± 0.11) [21]. Study participants´ fasting blood samples were collected in the morning and centrifuged immediately. Plasma samples were stored at -80ºC until measurement. The research protocol was approved by the local Clinical Research Ethical Committee at University Hospital of Jaén (Reference number PI031095). All subjects, patients and/or their legal’s guardians provided written informed consent.
2.2. L-Arginine, L-Citrulline and L-Ornithine Analysis
L-Arginine, L-citrulline and L-ornithine content were assayed by HPLC coupled to a fluorescence detection system, as previously described by us [27] with modifications. Briefly, plasma samples (40 µL) were deproteinated by ultrafiltration through a 10,000 molecular weight cut-off filter. The deproteinated plasma was precolumn derivatized with OPA reagent (o-phthaldialdehyde in borate buffer pH 9.5 containing 3-mercaptopropionic acid) and injected through a refrigerated Perkin-Elmer Series 200 automatic sample injector into a 150 x 3.9 mm Waters Resolve 5µ C-18 column. The mobile phase consisted of: (A) 53 mM sodium phosphate plus 1.2 mM EDTA (pH 6.3) and (B) 50/50 acetonitrile/water. A flow rate of 1 ml/min was maintained with a Perkin-Elmer Series 200 pump, using a gradient of solvent A from 90 to 0% in 30 min. The fluorescence detector (Perkin-Elmer Series 200a) was set at an excitation wavelength of 340 nm and an emission wavelength of 420 nm. Data were processed with the TotalChrom WorkStation ver. 6.3.1 software from Perkin-Elmer. Concentrations were expressed as n-moles of amino acid per ml. GABR is calculated as the ratio of L-arginine to the sum of L-ornithine + L-citrulline.
2.3. NOx Assay
The sum of nitrates and nitrites (NOx) was measured as the main metabolic end-products of NO and peroxinitrite anion (ONOOˉ). NOx (expressed as µmol/L) was determined as the concentration of nitrate plus nitrite in the plasma by a two-step process in a micro-titter plate format (Biovision Inc., Milpitas, California, USA), according to the manufacturer instructions. Firstly, nitrate is converted to nitrite utilizing nitrate reductase. Secondly, Griess reagents are used to convert nitrite to an azo compound. The amount of the azochromophore accurately reflects nitric oxide amount in samples.
2.4. Statistical Analysis
All values represent the mean of the individual determination ± Standard Error of the Mean (SEM). Data were analyzed by unpaired Student t test using IBM Pass V.19 software. ROC curves were analyzed and the Area Under the Curve (AUC), sensitivity and specificity calculated using MedCalc 12.2.1 package. Values of P<0.05 were considered significant.
3. RESULTS
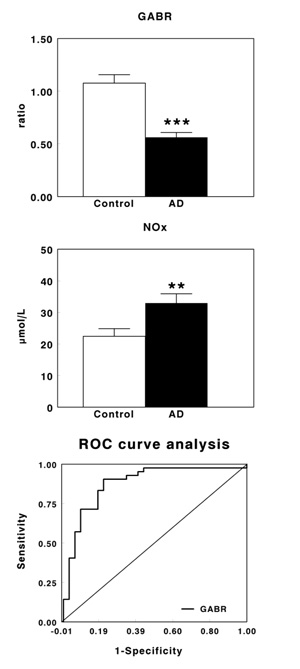
Table 1 shows plasma levels of arginine, citrulline and ornithine in healthy controls and AD patients. No significant changes were observed in arginine levels, whereas a significant increase in the levels of citrulline (P<0.001) and ornithine (P<0.01) were found in AD patients when compared with their corresponding healthy controls. Fig. (1A) shows plasma GABR in early-stage AD patients. GABR was significantly lower in AD patients than in control subjects (P<0.001), with a decrease of 47.8% in AD patients. On the contrary, plasma NOx levels increased significantly (P<0.01) in AD patients (Fig. 1B), being this increase by 46.9%. Fig. (1C) shows the ROC curve obtained for GABR. The optimal balance between sensitivity and specificity was found at a cut-off of 0.647. The overall diagnostic accuracy showed an AUC=0.877±0.0433 (P<0.0001), a sensitivity of 78.1 and a specificity of 90.5.
| Amino Acid | Control | Alzheimer Disease | Significance Level |
|---|---|---|---|
| L-Arginine | 82.73 ± 3.58 | 74.87 ± 4.34 | n.s. |
| L-Citrulline | 26.77 ± 2.21 | 61.30 ± 4.04 | P<0.001 |
| L-Ornithine | 65.30 ± 5.55 | 90.06 ± 8.75 | P<0.01 |
| Values are expressed in nmol/mL as mean ± SEM (n=46). | |||
4. DISCUSSION
The study of biochemical and physiological importance of L-arginine has been very wide for a long time. However, since the discovery of the multitude of functions of NO, the role of L-arginine has a growing interest, since this amino acid is the main physiological substrate for NO synthesis. In the nervous system, NO is involved in various signaling and defense functions, but can also be harmful if it is synthesized in excess or in the wrong place. Therefore, mechanisms that supply L-arginine to form NO in neuronal cells can be of great importance in the delicate balance between normal and pathological events that occur in the CNS, including AD [24, 28]. On the other hand, L-citrulline and L-ornithine are the two amino acids that originate after L-arginine catabolism. Therefore, it was introduced the concept of GABR as an indicator of NO synthesis capacity which is much better than the values of its metabolites only [6]. In fact, in our study, we have found no significant differences in plasma L-arginine levels between control subjects and patients with AD, whereas L-ornithine and L-citrulline levels were increased. Therefore, an important decrease in GABR was found in the plasma of AD patients. This decrease of about 48% correlates with the higher NOx levels also found in these patients (about 47%). Nitrate and nitrite are the stable end products of NO, and their levels have been used as an index of NO production [29]. The results showed here also agree with the data reported by several other authors who found increased levels of NO/NOx in AD patients when compared to healthy controls [30-32]. Also, Dildar et al. [33] have found increased NO plasma levels in probable AD patients but not in patients with vascular dementia, and this seems to be related to cognitive status. On the contrary, other authors did not find changes in plasma and/or CSF nitrate levels between AD patients and controls [34-36] or showed decreased levels of circulating NO with AD [37, 38]. These differences have been explained by the use of different methods of assay [32]. NO is a very labile molecule and it is difficult to directly assay in biological samples. Thus, NO data are usually reported as a measure of the total concentration of the products of oxidation of NO nitrite/nitrate (NOx). It is also possible the use of an inadequate number of patients and controls [32]. In fact, it has been described different results by the same authors in preliminary and later studies due to the number of subjects and controls [30, 37]. More recently, Liu et al. [39] described no changes in NOx levels in selected brain regions (superior frontal gyrus and anterior lobe of cerebellum) in AD patients, which appear to contradict with the AD- and/or age-related decrease in the total NOS activity found in these regions by radioenzymatic assay. They suggest that it could be possible the existence of more nitrate and nitrite produced endogenously (particularly from iNOS) in the oldest patients (over 80 years old). However, reduced or unchanged iNOS protein level found in AD patients or aged subjects does not support this idea. It has been also shown that NOx can also be dietarily obtained, in addition to endogenous NOS pathway [40]. In this sense, Milsom et al. [41] showed that dietary NOx intake restriction induced a significant reduction in nitrate and nitrite levels in the rat brains. Therefore, the certain contribution of dietary NOx to the brain NOx exists. Due to the blood-brain barrier does not function properly during AD and aging [42], there may be increased dietary and/or peripheral source of NOx in AD and advanced aged brains that must be also be taken into account. In any case, we describe for the first time the plasma GABR as an indicator of an altered L-arginine-NO pathway in AD and its putative use as a non-invasive biomarker. In fact, we have also evaluated the potential value of GABR as a diagnostic biomarker of AD in its earliest stages, using ROC curve analyses. In our hands, GABR showed a very good diagnostic accuracy in AD, and could be used for health screening of the elder population. Furthermore, the ideal AD biomarker would come from blood [43].
CONCLUSION
We can conclude that low plasma GABR levels in AD patients reflect that the L-arginine-NO pathway has turned towards NO in AD, probably producing nitroxidative stress, which promotes the development of neurodegenerative diseases such as AD. Furthermore, it could also be involved in altering some of its physiological functions such as the promotion of adequate cerebral blood flow, consolidation of memory processes, facilitation of long-term potentiation or maintaining the sleep-wake cycles. Finally, GABR is proposed as a putative useful biomarker of the disease.
CURRENT & FUTURE DEVELOPMENTS
Here, we have evaluated the potential value of GABR as a diagnostic biomarker of AD in its earliest stages, using a minimally invasive procedure. Employment of larger sample sizes and the differentiation from other related pathologies will clearly improve the utility of this procedure.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The present research was approved by the local Clinical Research Ethical Committee at University Hospital of Jaén (Reference number PI031095).
HUMAN AND ANIMAL RIGHTS
Animals were not used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All subjects, patients and/or their legal’s guardians provided written informed consent to participate in this research.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Supported by Junta de Andalucía through PAIDI BIO296 (currently CTS1039), Consejería de Salud through grant PI-0473-2009 and Consejería de Innovación, Ciencia y Empresa through grant P07-CVI-02758.


