All published articles of this journal are available on ScienceDirect.
Vitamin D Levels, Periodontal Parameters, and VDR Gene Polymorphism in Dental Implant Osseointegration Outcomes: A Case-Control Study in Egyptians
Abstract
Introduction
Failure of dental implants due to inadequate osseointegration continues to pose a major clinical issue, with increasing evidence suggesting that systemic and genetic factors play a role in the outcomes. It is proposed that vitamin D deficiency and genetic variations, such as those in the vitamin D receptor (VDR) gene, may affect bone metabolism and the success of implants. This case-control study aimed to evaluate the association of serum vitamin D levels and VDR gene polymorphism (SNP rs228570) with dental implant osseointegration success or failure.
Methods
A case-control study with 42 cases of implant failure and 42 controls with successful osseointegration was conducted in patients aged 31–60 years. Sociodemographic, clinical, and periodontal parameters were analyzed, and VDR SNP rs228570 was genotyped using real-time PCR, and serum vitamin D, TNF, and IL-6 levels were measured. Statistical analysis was performed using SPSS v28.0, with univariate and multivariable logistic regression with significance (p < 0.05).
Results
No significant association was found between VDR gene polymorphism (SNP rs228570) and implant failure (p > 0.05). However, there is a significant association between higher vitamin D levels and successful osseointegration. Vitamin D levels were significantly higher in the successful group (36.85 ng/ml ± 11.55) compared to the failed group (17.03 ng/ml ± 9.16) (p < 0.001). Clinical parameters revealed significant differences, with the successful group showing lower bleeding on probing (BOP) at 25.15% (SD ± 5.31) compared to 42.46% (SD ± 7.59) in the failed group (p < 0.001) and a shallower probing depth of 2.21 mm (SD ± 0.85) compared to 5.62 mm (SD ± 1.32) in the failed group (p < 0.001). Biochemical markers such as TNF and IL-6 did not show significant differences between the groups, P-value = 0.181 and 0.186, respectively.
Discussion
The study highlights the importance of vitamin D levels and clinical parameters such as BOP and PD in predicting osseointegration outcomes.
Conclusion
The study highlights the importance of vitamin D levels and clinical parameters such as BOP and PD in predicting osseointegration outcomes. Although VDR gene polymorphism showed no significant association with implant failure, higher vitamin D levels were positively correlated with successful osseointegration. These findings suggest that optimizing vitamin D levels and managing peri-implant health may improve dental implant success rates, emphasizing clinical over genetic predictors.
1. INTRODUCTION
Dental implants are widely recognized as a durable and visually appealing option for replacing missing teeth, making them a fundamental aspect of restorative dentistry [1]. The success of these implants largely depends on osseointegration, which is the direct and functional connection between the implant surface and the surrounding bone tissue [2]. Achieving this bond requires careful consideration of multiple factors, including surgical techniques, prosthetic design, biomaterial properties, and patient-specific conditions [3].
Recent research emphasizes the role of systemic health factors, particularly nutrition, in influencing osseointegration [4]. Among these, vitamin D, a fat-soluble nutrient critical for bone health, stands out. It regulates calcium and phosphate metabolism, promoting bone remodeling and repair by stimulating osteoblasts and controlling osteoclasts [5]. Adequate vitamin D levels are essential for maintaining bone density and supporting the healing process after dental implant procedures. Studies suggest that optimal vitamin D levels can enhance bone formation around implants, leading to faster and more effective osseointegration [6].
Despite its importance, vitamin D deficiency is a global health concern, affecting approximately 1 billion people worldwide [7]. This deficiency is particularly common among older adults, who represent a significant proportion of dental implant recipients [8]. However, the relationship between vitamin D levels and osseointegration remains unclear. While some studies indicate that sufficient vitamin D levels improve implant integration and bone healing, others report no significant association [9].
Emerging evidence suggests that genetic factors may play a role in the success of dental implants. Variations in the Vitamin D Receptor (VDR) gene, which regulates the biological effects of vitamin D, have been linked to conditions such as osteoporosis and periodontal disease, both of which involve bone loss [10, 11]. One specific polymorphism in the VDR gene, FokI (rs228570 or rs10735810), alters the length of the protein, potentially affecting its biological activity. The C allele produces a shorter, more active protein, while the T allele results in a longer, less active form [12, 13]. These genetic variations may influence vitamin D metabolism and, consequently, the success of osseointegration [14]. Given the importance of bone healing in dental implant success, this study aimed to investigate the role of vitamin D levels and VDR gene polymorphisms in osseointegration among a sample of Egyptian adults. The study also explored the potential link between genetic variations in the VDR gene and the success or failure of dental implants.
2. METHODS
2.1. Study Design
The study was designed as a case-control study, with cases defined as subjects experiencing failed osseointegration and controls as those with successful osseointegration. Participants were recruited from the dental implant clinic at the British University in Egypt between October 2022 and June 2023.
2.2. Participants
2.2.1. Ethics Approval and Consent to Participate
This case–control study was approved by the Ethics Committee on Research of the British University in Egypt (BUE), No. 0003772/10—Protocol No. 323. The study was performed as a single blind study where the data analyst was blinded, simply by using a neutral generic identifier to label the case group A and the control group B. Moreover, random.org was used to assign a random code number to each participant to ensure the analyst receives de-identified data with neutral group labels.
2.2.2. Inclusion Criteria
Participants who presented to the dental implant clinic in the British University in Egypt to receive dental implants with no known uncontrolled medical conditions and who have not received any prior bone augmentation procedures from the period of October 2022 to June 2023.
2.2.3. Exclusion Criteria
Exclusion criteria included participants with uncontrolled medical conditions, medications affecting healing or blood clotting, uncontrolled periodontal disease, osteoporosis, blood clotting disorders, active cancer treatment, smoking, age below 22 or above 80, and known congenital bone disorders.
2.3. Sample Size Calculation
A power analysis was designed to have adequate power to apply a two-sided statistical test of the null hypothesis that there is no difference between the tested groups. By adopting an alpha (α) level of 0.05 (5%), a beta (β) level of 0.2 (i.e., power=80%), and an effect size (d) of 9.8%, calculated based on the results of a previous study [15], the predicted total sample size n was found to be 84 cases, i.e., 42 cases per groups. G-Power 3.1.9.7 was utilized to calculate the sample size.
2.4. Data Collection
Participants provided detailed personal, medical, and dental histories. Informed consent was obtained from all participants before any dental implant surgery. Gingival crevicular fluid (GCF) was collected using paper point adsorption and diluted in saline. Patients were followed up for three and six months post-surgery to assess osseointegration. Based on osseointegration status, patients were classified as responders or non-responders, and samples were analysed for VDR gene variants using allele discrimination.
The study protocol complied with the guidelines of the Helsinki Declaration; all the steps of this study were explained in full detail to every participating subject who signed an informed consent. After taking a proper full medical and dental history, an oral examination was performed. Bleeding on probing BOP percentages were noted. Following the selection of the GCF sampling locations, appropriate drying was carried out, and then, to prevent saliva contamination, these locations were carefully isolated using cotton rolls. The pocket or sulcus was filled with a 2 x 9 mm filter paper until a slight resistance was found. The paper was left for 30 seconds to allow for the collection of an appropriate amount of GCF. Afterwards, the paper was withdrawn and placed in a plastic Eppendorf tube and then kept frozen at −20 ºC until assayed for gene polymorphism analysis. Any paper with any blood contamination was discarded to avoid any errors in the assessment of Vit. D level [16]
2.4.1. Measurement of Serum Vitamin D3
The measurement of Vitamin D3 (25OHD) in serum was performed using venous blood collection, 5 mL, using a sterile disposable needle, performed by a trained nurse. The 25-hydroxy vitamin D (25OHD) test was used to assess vitamin D3 level. Methodology and reference range for vitamin D measurement: The standard Enzyme-Linked Immuno-Sorbent Assay was used to analyse 5-hydroxy vitamin D3 (EIA-3153 DRG International Inc., USA) [14]. The determination of absorption was measured with an ELISA reader, SATAT FAX 3300. Serum 25 OH levels of 35 ng/mL or higher were considered normal, and less than 35 ng/mL were considered low.
2.4.2. Measurement of Tumour Necrosis Factor-alpha TNF-Alpha
Serum Tumour necrosis factor-alpha was measured by TNF-Alpha ELISA Assay Kit, Immunodiagnostic, Germany [17]. Interleukin-6 (IL-6) levels were measured using the Roche Cobas e411Roche Diagnostics GmbH, Mannheim, Germany. Both markers were analysed according to manufacturer's instructions using Eliza washer and reader stat fax 3300.
2.4.3. Gene Polymorphism Analysis
Extracting DNA: Following the manufacturer's instructions, the DNA was extracted using the QIA amp® DNA Blood Mini Kit QIAGEN GmbH, Hilden, Germany. The extracted DNA concentration was determined using the Nano Drop® ND-1000 Spectrophotometer Nano Drop Technologies Inc., Washington, DC, USA. The absorbance ratio of isolated DNA at 260/280 nm was 1.7–1.9.
2.4.4. Genotyping for VDR rs228570
Genotyping for VDR SNPs rs228570 was performed using real-time polymerase chain reaction RT-PCR with the TaqMan® allelic discrimination assay on the Applied Biosystems Step OneTM Real-Time PCR System (Thermal Cycling Block, Singapore), in accordance with the manufacturer's guidelines.
2.5. Statistical Analysis
Categorical data were presented as frequencies and percentages and analyzed using chi-square tests. Numerical data were summarized as means, standard deviations (SD), medians, and interquartile ranges (IQR). Normality and variance homogeneity were assessed using Shapiro-Wilk’s and Levene’s tests, respectively. Non-normally distributed data were analyzed using the Mann-Whitney U test. A ridge regression model was used for multivariable analysis due to multicollinearity among variables, as indicated by Variance Inflation Factors (VIF). Predictors were standardized, and the optimal penalty parameter (Lambda) was selected using Generalized Cross-Validation (GCV). Statistical significance was set at p < 0.05, and all analyses were performed using R statistical software.
3. RESULTS
A total of 84 patients were included, with 42 classified as successful cases and 42 as failed cases based on osseointegration criteria. Significant differences were observed between the two groups in terms of bleeding on probing (BOP), probing depth (PD), and vitamin D levels, while variables such as age, sex, and smoking status showed no significant differences.
3.1. Univariate Analysis
Table 1 provides an overview of the univariable associations between various parameters and osseointegration outcomes.
3.1.1. Demographic Variables
The mean age of patients in the successful group was 46.32 years (SD ± 7.86), compared to 45.19 years (SD ± 8.54) in the failed group, with no significant difference (p = 0.415). Sex distribution also showed no significant association, with 46.81% of successful cases being male compared to 54.05% in the failed group (p = 0.510). Smoking status was similarly unrelated to osseointegration outcomes, with 59.57% of successful cases being non-smokers compared to 51.35% in the failed group (p = 0.451).
3.1.2. Clinical Parameters
Significant differences were observed in BOP and PD between the two groups. The successful group had a mean BOP percentage of 25.15% (SD ± 5.31), significantly lower than the 42.46% (SD ± 7.59) in the failed group (p < 0.001). The probing depth was shallower in the successful group (2.21 mm ± 0.85) compared to the failed group (5.62 mm ± 1.32), with a p-value of <0.001. These findings suggest that higher BOP and greater probing depth are linked to implant failure.
3.1.3. Biochemical Parameters
Vitamin D levels were significantly higher in the successful group (36.85 ng/ml ± 11.55) compared to the failed group (17.03 ng/ml ± 9.16), with a p-value of <0.001. This indicates that higher vitamin D levels are associated with successful osseointegration. Other biochemical markers, such as TNF and IL-6, did not show significant differences between the groups. The mean TNF level was 30.34 pg/mL (SD ± 12.49) in the successful group and 26.11 pg/mL (SD ± 8.92) in the failed group (p = 0.181). Similarly, IL-6 levels were 26.79 pg/mL (SD ± 9.67) in successful cases and 30.65 pg/mL (SD ± 12.56) in failed cases (p = 0.186).
3.1.4. Gene Polymorphism
No significant associations were found between gene polymorphisms (CT, TT, and CC) and osseointegration outcomes. The CT polymorphism was present in 55.32% of successful cases and 32.43% of failed cases (p = 0.106). The TT polymorphism was observed in 10.64% of successful cases and 18.92% of failed cases, while the CC polymorphism was found in 34.04% of successful cases and 48.65% of failed cases.
3.2. Multivariable Analysis
A ridge regression model was used to control for potential confounders and further evaluate the predictors of osseointegration outcomes. The optimal penalty parameter (Lambda) was determined to be 3.44 using generalized cross-validation (GCV). The results are summarized in (Table 2, Figs. 1 and 2).
| Variables | Cases (Success) | Controls (Failure) | Test Statistics | p-value |
|---|---|---|---|---|
| Age (years) | 46.32 ± 7.86 | 45.19 ± 8.54 | 960.00 | 0.415 |
| Sex [n (%)] | ||||
| - Male | 22 (46.81%) | 20 (54.05%) | 0.43 | 0.510 |
| - Female | 25 (53.19%) | 17 (45.95%) | ||
| Smoking [n (%)] | ||||
| - No | 28 (59.57%) | 19 (51.35%) | 0.57 | 0.451 |
| - Yes | 19 (40.43%) | 18 (48.65%) | ||
| BOP (%) | 25.15 ± 5.31 | 42.46 ± 7.59 | 1701.00 | <0.001* |
| Probing Depth (mm) | 2.21 ± 0.85 | 5.62 ± 1.32 | 1726.00 | <0.001* |
| Gene Polymorphism [n (%)] | ||||
| - CT | 26 (55.32%) | 12 (32.43%) | 4.48 | 0.106 |
| - TT | 5 (10.64%) | 7 (18.92%) | ||
| - CC | 16 (34.04%) | 18 (48.65%) | ||
| Vitamin D (ng/ml) | 36.85 ± 11.55 | 17.03 ± 9.16 | 1585.50 | <0.001* |
| TNF (pg./ml) | 30.34 ± 12.49 | 26.11 ± 8.92 | 1017.50 | 0.181 |
| IL-6 (pg./ml) | 26.79 ± 9.67 | 30.65 ± 12.56 | 1016.00 | 0.186 |
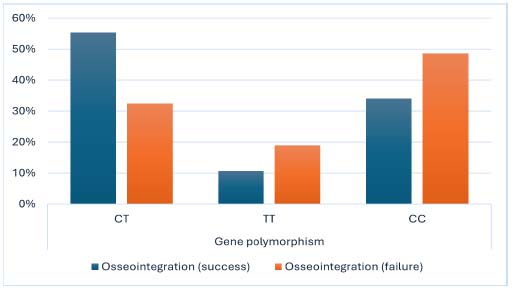
Distribution of VDR gene polymorphism (SNP rs731236) genotypes in implant failure (Case) and successful osseointegration (Control) groups.
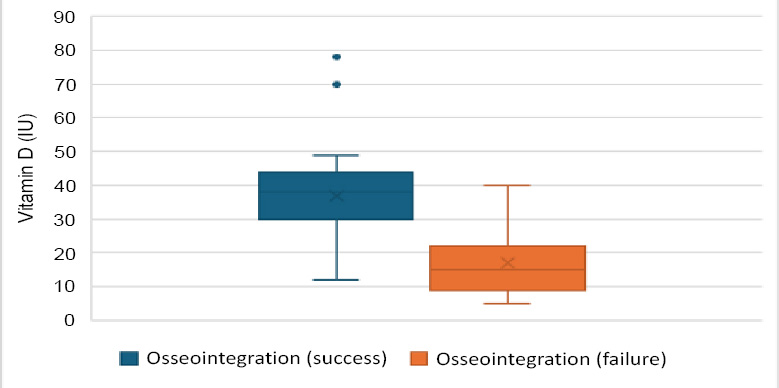
Box plot for vitamin D serum levels in implant failure and success groups.
| Variable | Coefficient | Standard Error | Test Statistic | p-value |
|---|---|---|---|---|
| Age | 0.34 | 0.17 | 1.98 | 0.051 |
| Sex (female) | -0.18 | 0.17 | 1.06 | 0.288 |
| Smoking (yes) | -0.19 | 0.17 | 1.12 | 0.262 |
| BOP | 1.93 | 0.22 | 8.91 | <0.001* |
| PD | 2.30 | 0.22 | 10.70 | <0.001* |
| Gene polymorphism CT | 0.01 | 0.18 | 0.04 | 0.968 |
| Gene polymorphism TT | -0.17 | 0.18 | 0.95 | 0.344 |
| Vitamin D | -0.71 | 0.21 | 3.42 | 0.001* |
| TNF | -0.41 | 0.18 | 2.24 | 0.025* |
| IL-6 | 0.00 | 0.19 | 0.02 | 0.982 |
3.2.1. Significant Predictors of Osseointegration Outcomes
The multivariable analysis confirmed that BOP and PD were significantly associated with implant failure, with coefficients of 1.93 (p < 0.001) and 2.30 (p < 0.001), respectively. This supports the univariable findings that higher BOP and deeper probing depths are linked to unsuccessful osseointegration. Vitamin D levels also emerged as a significant predictor, with a coefficient of -0.71 (p = 0.001), indicating that lower vitamin D levels are associated with higher failure rates. Additionally, TNF levels were significant, with a coefficient of -0.41 (p = 0.025), suggesting that lower TNF levels may contribute to unsuccessful outcomes.
3.2.2. Non-significant Predictors
Age, sex, smoking status, IL-6 levels, and gene polymorphisms did not show significant associations with osseointegration outcomes in the multivariable model. Age had a coefficient of 0.34 (p = 0.051), indicating a marginal association, while sex and smoking status had coefficients of -0.18 (p = 0.288) and -0.19 (p = 0.262), respectively. Gene polymorphisms and IL-6 levels also showed no significant associations, with p-values of 0.968 and 0.982, respectively.
4. DISCUSSION
This study aimed to explore the complex interplay of clinical, biochemical, and genetic factors affecting osseointegration outcomes in dental implant surgery. The findings offer critical insights into the predictors of successful osseointegration, emphasizing the importance of clinical indicators such as bleeding on probing (BOP) and probing depth (PD), as well as biochemical markers like vitamin D levels. The study challenges the relevance of demographic and genetic factors in influencing implant success.
The results in this study highlight the pivotal role of clinical parameters in predicting osseointegration success. BOP and PD were strongly associated with implant outcomes. The successful group demonstrated significantly lower BOP percentages and shallower probing depths compared to the failed group, with p-values indicating robust statistical significance (p < 0.001). These findings align with existing research linking periodontal health to implant stability [17]. Elevated BOP often signals inflammation or peri-implant disease, which can hinder osseointegration [18]. Increased probing depths may reflect bone loss or poor tissue adaptation, further contributing to implant failure [19]. These results underscore the need for clinicians to closely monitor these parameters during both preoperative and postoperative phases to reduce the risk of failure [20].
A key finding of this study is the significant association between vitamin D levels and osseointegration outcomes. Patients in the successful group had significantly higher vitamin D levels compared to those in the failed group (p < 0.001). This aligns with evidence that vitamin D plays a vital role in bone metabolism and immune function, both of which are critical for successful osseointegration [21]. Vitamin D enhances calcium absorption and promotes osteoblast activity, facilitating bone formation around the implant [22]. Recent work demonstrated that preoperative vitamin D optimization reduced implant failure rates by 30% in deficient populations. The potential benefits of vitamin D supplementation in improving osseointegration outcomes, particularly in populations at risk of deficiency, warrant further exploration [23].
While other biochemical markers, such as TNF and IL-6, did not show significant differences between the groups, the multivariable analysis revealed a marginal significance for TNF levels (p = 0.025). This suggests that lower TNF levels may be linked to higher success rates, potentially indicating a reduced inflammatory response [24]. However, the lack of significant differences in IL-6 levels highlights the complexity of inflammation’s role in osseointegration, necessitating a cautious interpretation of these findings [25]. TNF’s role may be context-dependent, influenced by comorbidities such as diabetes, which were excluded in our cohort.
Contrary to expectations, the analysis of gene polymorphisms revealed no significant associations with osseointegration outcomes. The presence of CT, TT, and CC polymorphisms did not correlate with success or failure rates, raising questions about the relevance of genetic factors in this context [26]. While genetic predispositions can influence various biological processes, the absence of significant findings in this study suggests that clinical and biochemical factors may play a more dominant role in determining osseointegration success [27]. This challenges the notion that genetic screening should be a routine part of pre-implant evaluations, shifting focus toward modifiable risk factors such as clinical parameters and vitamin D levels [28].
The demographic variables assessed, including age, sex, and smoking status, did not show significant associations with osseointegration outcomes. This aligns with some studies that have reported similar findings, suggesting these factors may be less influential than previously assumed [29, 30]. Although age showed a marginal association (p = 0.051), it did not reach conventional significance levels. A recent study showed no age-related trends in a multicentre cohort, further questioning the clinical emphasis on demographic predictors [31].
5. LIMITATIONS
Firstly, the single-center design (British University in Egypt) may limit generalizability to populations with differing genetic or environmental profiles. Secondly, the sample size (n = 84), while adequate for detecting clinical predictors, may lack the power to identify subtle genetic associations. Thirdly, unmeasured confounders such as socioeconomic status and dietary habits could influence outcomes [32].
6. IMPLICATIONS
These findings support actionable clinical and policy changes:
a. Preoperative vitamin D screening: Implement thresholds (<30 ng/mL) to trigger supplementation, reducing failure risk [33-37].
b. Demographic deprioritization: Shift focus from non-modifiable factors such as age and smoking to biomarkers and periodontal health.
CONCLUSION
This study advances our understanding of the factors influencing osseointegration outcomes in dental implant surgery. The significant associations between clinical parameters (BOP and PD) and biochemical factors (vitamin D levels) highlight the importance of comprehensive patient assessment and management strategies.
Societal Impact:
This study supports public health initiatives to promote vitamin D sufficiency and periodontal health awareness, especially in high-risk populations, which could reduce implant failure rates and associated healthcare costs. By prioritizing accessible screening and preventive care, this work supports equitable oral health outcomes and improves the socioeconomic burden of implant-related complications.
Future Directions:
Longitudinal studies are needed to establish the connection between vitamin D optimization, periodontal management, and long-term implant success. Also, multicentre trials across diverse populations should validate this threshold (vitamin D ≥30 ng/mL) and explore interactions with unmeasured confounders such as dietary habits or comorbidities. The BOP threshold should be studied for implant success. Continued research in this area will be essential to refine clinical protocols and enhance the predictability of dental implant procedures globally.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: A.D., M.I., A.A.M.: Study conception and design: A.A.M., O.E., M.M., A.R., M.A., A.A., R.E., A.b.A., H.H., A.G.A., H.H.E., R.S., S.A., K.N.: Data collection; K.N.: Data analysis or interpretation; A.D., M.I., A.A.M., O.E., M.M., A.R., M.A., A.A., R.E., A.b.A., H.H., A.G.A., H.H.E, R.S., S.A., K.N.: Methodology; A.D., M.I., A.A.M.; Investigation; A.D., M.I., A.A.M., S.A.: Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| BOP | = Bleeding on Probing |
| ELISA | = Enzyme-Linked Immuno-Sorbent Assay |
| GCF | = Gingival Crevicular Fluid |
| GCV | = Generalized Cross Validation |
| IQR | = Interquartile Range |
| PD | = Probing Depth |
| RT-PCR | = Real-time Polymerase Chain Reaction |
| SNP | = Single Nucleotide Polymorphism |
| TNF | = Tumor Necrosis Factor |
| VIF | = Variance Inflation factor |
| VDR | = Vitamin D Receptor |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This case–control study was approved by the Ethics Committee on Research of the British University in Egypt (BUE), No. 0003772/10—Protocol No. 323.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent to participate in the study was obtained from all participants.
AVAILABILITY OF DATA AND MATERIALS
The data of current study are available from corresponding author, [S.A.E], on a reasonable request.
ACKNOWLEDGEMENTS
Declared none.


