All published articles of this journal are available on ScienceDirect.
Study of Gender-based Changes in P53 in Preeclampsia
Abstract
It aims to compare the levels of p53 in maternal and umbilical cord venous samples of healthy pregnant and preeclamptics.
Background:
Preeclampsia is a leading cause of both maternal morbidity and neonatal mortality. The etiology and pathogenesis of preeclampsia are not yet fully understood. Apoptosis during pregnancy develops due to multiple different mechanisms.
No studies are available in the literature documenting any association between fetal sex and p53 levels; also, the status of p53 in cord blood is unclear.
Objective:
Hence the study was designed to compare p53 levels in maternal and umbilical cord venous samples to study both maternal and fetal aspects of preeclampsia.
Methods:
The present study was conducted in 30 normotensive, primigravida women and 30 primigravida preeclamptics (age and gestation matched) with a singleton pregnancy. Serum p53 analysis was carried out in maternal serum and cord blood by solid phase sandwich enzyme-linked immunosorbent assay (Elisa kit).
Results:
In the present study, maternal and cord p53 levels in preeclamptics were higher. The cord blood p53 levels were significantly higher in preeclamptic mothers with female babies than in preeclamptic mothers with male babies.
Conclusion:
These findings indicate a definitive role of apoptosis in the pathogenesis of preeclampsia and may be useful in diagnosing patients with preeclampsia and identifying future natal, perinatal and maternal risks.
Demonstrating these gender-based changes in p53 levels suggests an active contribution of the placenta in metabolism during pregnancy.
1. INTRODUCTION
Preeclampsia is a leading cause of both maternal morbidity and neonatal mortality. Numerous theories on the pathophysiological mechanisms have been investigated; nevertheless, the etiology and pathogenesis of preeclampsia are not yet fully understood [1, 2]. Endothelial dysfunction is a hallmark of preeclampsia. Additionally, reduced invasion of the trophoblast and placental hypoxia cause placental oxidative stress [2]. The placenta is a unique fetally-derived complex organ that continues to develop and change throughout gestation and is discarded immediately after birth. It provides nutrition to the developing fetus, removes waste products, has endocrine functions and protects the maternal immune system [3].
The pregnancy pathology is characterized by an altered balance between proliferation and apoptosis of villous trophoblast resulting in a dysregulated release of material from the syncytiotrophoblast into maternal blood [4, 5]. Poor placental blood flow subsequently promotes trophoblast cell death via apoptosis, and p53 is a key protein in the regulation of apoptosis. Under normal conditions, there is a perfect balance between vascular cellular proliferation and death; in arterial hypertension, there is increased proliferation and apoptosis, and a new balance is set at a higher level,” and remodeling of the wall occurs, causing reduced vessel lumen [6, 7]. Data regarding a possible association between fetal sex and levels of p53 are not available in the literature, and the status of p53 in cord blood is unclear. The present study was designed to compare p53 levels in maternal and umbilical cord venous samples to study both maternal and fetal aspects of preeclampsia.
2. MATERIALS AND METHODS
The present study was conducted on sixty pregnant women admitted to the Obstetrics ward. Informed consent has been taken from all the patients. These women were subdivided as GROUP I (controls, n=30) comprised of normotensive pregnant women (primigravida) with singleton pregnancy at the time of delivery and GROUP II (study, n=30) of preeclamptic women (age and gestation matched primigravida with singleton pregnancy) and systolic blood pressure reading ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg and/or proteinuria at the time of delivery. Women with any history of chronic hypertension, metabolic disorders or high-risk factors such as severe anemia, diabetes, renal disease, heart disease, or malignancies were excluded. The maternal venous blood sample was collected from the antecubital vein aseptically, and six ml of cord blood was collected in vacutainer tubes from the umbilical cord (placental end) immediately after delivering the baby. Serum was separated (by centrifugation), routine investigations were carried out on the same day as per standard methods, and serum was preserved at -20°C until further analysis of special investigations. Both maternal serum and cord blood serum p53 analysis was carried out by human p53 ELISA KIT (Diaclone kit: based on solid phase sandwich Enzyme Linked-Immuno–Sorbent Assay) [8, 9].
All the analyses were performed using the statistical package (SPSS 20). Results were expressed as mean values, and the Standard deviation Unpaired ‘t-test and two-tailed Pearson correlation test between variables were applied.
3. RESULTS
The mean age of the mothers at the time of delivery in both groups was comparable (29.10 + 2.23 years and 27.36 +4.10 years, respectively. The gestational age of mothers ranged from 34-41 weeks at delivery. The majority of women had a full-term vaginal delivery (FTND) in both categories, fourteen (46.66%) in normotensive mothers and twelve (40%) in preeclamptic women. The birth weight was significantly lower in babies who delivered to preeclamptic women as compared to their normotensive counterparts [2.86 ± 0.29 kg and 2 .63 ± 0 .27 kg, respectively, (p =0.0029, p<0.05)]. The majority of the babies were born with a good Apgar Score. In the present study, in the preeclamptic group, 13 female babies were delivered at a gestational age of 34-38 weeks, and 5 female babies were delivered at gestation >38weeks, whereas, in normotensive women, 8 female babies at a gestation age of 34-38 weeks and 7 female babies delivered at a gestational age of >38weeks. In the present study, in preeclamptics, the male: female ratio was 0.38 at gestation 34-38 weeks and 1.4 at gestation >38 weeks, whereas in normotensives, the ratio was 0.11 at gestation 34-38 weeks and 2.33 at gestation >38 weeks. There was no delivery with gestation <34. Male preponderance has been observed in the study at a gestation of >38 weeks in both normotensive and preeclamptic groups. However, at a gestation of 34-38 weeks, female preponderance has been observed in both the groups normotensive and preeclamptics.
Table 1 depicts the clinical characteristics of both groups. Hemoglobin and blood glucose levels were insignificant in the preeclamptic group compared to the normotensive group. Other biochemical parameters showed a significant difference in preeclamptics compared to the normotensive group. Table 2 depicts p53 levels in maternal serum were higher in preeclamptic women (2.75 ± 0.96 U/ml) as compared to normotensive pregnant women (2.10 ± 0 .98 U/ml), and the difference was statistically significant [(p=0.011); p<0.05). Higher levels of cord blood p53 levels were observed in preeclamptic women (3.70 ± 1.51 U/ml) as compared to normotensive counterparts (2.84 ± 1.56 U/ml), and the difference was statistically significant [(p=0 .03); p<0 .05). The proportion of p53 levels in cord blood as compared to maternal serum in normotensives and preeclamptics was 1.34 in normotensive and 1.35 in preeclamptic women, and they were comparable. In normotensive mother with male baby, maternal serum p53 levels were higher (2.48 ± 1.23 U/ml) as compared to normotensive mother with female baby (1.72 ± 0.36 U/ml) and the difference was statistically significant [(p<0.05), p=0.03, Table 2, Fig. 1). Cord blood p53 levels in preeclamptic women with female baby, were significantly higher (4.26 ± 1.61 U/ml) when compared with normotensive women with female baby (2.60 ± 1.36 U/ml) and the difference was statistically significant [(p=0 .0032); p<0.05). p53 levels in cord blood were significantly higher in preeclamptics with female babies (4.26 ± 1.61 U/ml) as compared to preeclamptic mothers with male babies (2.88 ± 0.89 U/ml), but the difference was statistically significant (p=0 .005), p<0.05, (Table 2, Fig. 1). On comparing, gender of the baby with gestational age, a negative correlation was identified in normotensive women in the present study (r=-0 .16, p=0 .02, p< 0.05). However, in preeclamptic women, this was inversed (r= -0 .06, p>0.05).
4. DISCUSSION
In cell proliferation and differentiation, apoptosis plays an important role and is regulated by several genes [10]. Low levels of apoptosis in placental villi and decidual tissues is a normal physiological phenomenon. High p53 expression levels may cause elevated apoptosis processes, leading to pathological alterations in the placenta and abnormalities during pregnancy.
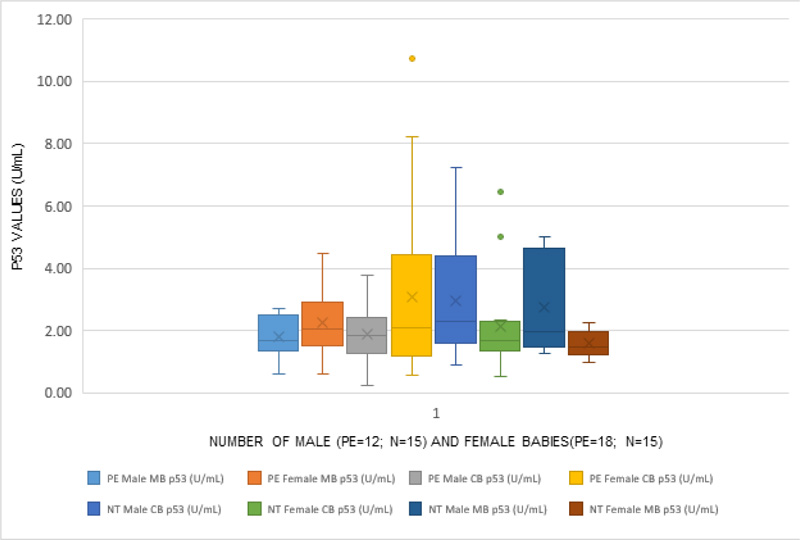
(PE= Preeclampsia, N=Normotensive, MB=Maternal Blood, CB= Cord Blood).
| Parameters |
Normotensive Pregnant Women (Group I) (n=30) |
Preeclamptic Women (Group II) (n=30) |
|---|---|---|
| Hemoglobin (g/dl) | 10.53 ± 1.24 | 9.97 ± 1.48* |
| Blood sugar (mg/dl) | 90.5 ± 5.89 | 92.56 ± 8.11* |
| Blood urea (mg/dl) | 21.06 ± 6.57 | 42.03 ± 9.87*** |
| Serum creatinine (mg/dl) | 0.56 ± 0.09 | 0.845 ± 0.16*** |
| Serum uric acid (mg/dl) | 3.42 ± 0.74 | 5.62 ± 1.02*** |
| Serum calcium (mg/dl) | 8.97 ± 0.78 | 7.86 ± 0.96*** |
| Serum bilirubin (mg/dl) | 0.59 ± 0.11 | 0.64 ± 0.09** |
| AST/SGOT (U/L) | 29.63 ± 8.04 | 51.66 ± 9.97*** |
| ALT/SGPT (U/L) | 15.76 ± 3.63 | 19.33 ± 3.31*** |
| Serum alkaline phosphatase (U/L) | 58.53 ± 8.62 | 192.46 ± 10.91*** |
| Total protein (g/dl) | 7.03 ± 0.44 | 5.68 ± 0.33*** |
| Serum albumin (g/dl) | 3.97 ± 0.18 | 3.11 ± 0.28*** |
** p<0.05 significant as compared to group I
***p<0.001 highly significant compared to group I
| Serum p53 Levels (U/ml) | Normotensive Women | Preeclamptic Women | p-value | |
|---|---|---|---|---|
| Total | ||||
| Maternal serum p53 levels | 2.10 ± 0.98 | 2.75 ± 0.96 | 0.032 | |
| Cord blood p53 levels | 2.84 ± 1.56 | 3.70 ± 1.51 | 0.005 | |
| Male babies | ||||
| Maternal serum p53 levels | 2.48 ± 1.23 | 2.35 ± 0.41* | 0.70 | |
| Cord blood p53 levels | 3.07 ± 1.76 | 2.88 ± 0.89* | 0.71 | |
| Female babies | ||||
| Maternal serum p53 levels | 1.72 ± 0.36 | 3.02 ± 1.21*** | 0.00014 | |
| Cord blood p53 levels | 2.60 ± 1.36 | 4.26 ± 1.61** | 0.0032 | |
** p<0.05 significant as compared to group I counterparts
*** p<0.001 highly significant as compared to group I counterparts
Many studies have shown that a high level of apoptosis in the chorionic villi and decidua is associated with RSA, revealing that apoptosis may be one of the causes of recurrent spontaneous abortions [10, 11]. p53, a key cell cycle regulator, is important in numerous biological processes, namely, cell cycle, DNA repair, differentiation, and apoptosis. p53 has been reported to be expressed abnormally in chorionic villi and decidua of females with hydropic, spontaneous or missed abortions [12]. In preeclampsia, exaggerated apoptosis of villous trophoblast of placental villi occurs. p53 being a critical regulator of apoptosis, excessive apoptosis in PE is mediated by abnormal expression of proteins participating in the p53 pathway, and that modulation of the p53 pathway alters trophoblast apoptosis. In the present study, p53 levels in maternal serum were significantly higher in preeclamptic women as compared to normotensive pregnant women (p<0 .05). Also, cord blood p53 levels were higher in preeclamptic women as compared to normotensive pregnant women (p<0.05). These findings lend support to the involvement of p53 in pathogenesis. Certain studies have reported that the male gender of the fetus is associated with an increased risk of developing pre-eclampsia in the mother [13]. However, it was reported that the male-to-female birth ratio decreased in preeclamptics who had preterm delivery [13]. The reason for this discrepancy is not clear. No such comparison was possible as there was no preterm delivery in the study. The placentation and dysfunctional modelling of uteroplacental arteries during pregnancies predispose to preeclampsia and a sex-specific susceptibility to embryonic implantation responsible for sex dimorphic differences in preeclampsia. This suggests a possible role of oxidative stress in developing preeclampsia during pregnancy, severe dyslipidemia and even future risk of cardiovascular disease in mother and child [9]. A study investigated whether gender-associated risk changed with gestational age and observed that when all gestational ages were evaluated, newborn male gender was associated with increased odds ratios [13]. The higher male proportion reported in literature holds for stillborn and spontaneously aborted fetuses [9]. Gender of the baby showed a significant negative correlation with gestational age in normotensive women in the present study (r=-0 .16, p=0.02, p<0.05). However, in preeclamptic women, a non-significant negative correlation was noted (r= - 0.06, p>0.05). In contrast, some studies have documented the association of male fetal gender with a lower risk for pre-eclampsia, thus, leading to preterm delivery [14]. Also, a Norwegian population-based study has shown decreased male-to-female birth ratio (M/F ratio) in preterm births associated with pre-eclampsia [9]. In the present study, in preeclamptics, the male: female ratio was noted to be 0.38 at gestation 34-38 weeks and 1.4 at gestation >38weeks whereas, in normotensives, the ratio was 0.11 at gestation 34-38 weeks and 2.33 at gestation >38 weeks. Since there was no delivery with gestation <34 weeks, no male-to-female ratio could not be calculated. The present study observed the male preponderance at the gestation of >38 weeks in both normotensive and preeclamptic groups. However, at the gestation of 34-38 weeks, female preponderance has been observed in both the groups. Also, the development of preeclampsia early in pregnancy was associated with preterm birth, and there was an increased likelihood of female fetuses in these cases. Preeclampsia developing later in gestation has been associated with the male fetal gender in most cases. This indicates that mechanisms for the onset of pre-eclampsia at early and later stages might differ [14], and the risk of developing these conditions can also be affected by the fetal gender. This observation might put new insights into pathophysiological mechanisms, as evident by findings of the present study and reports available in the literature and future studies. Thus, the gender perspective of the fetus and newborn may give new insight into the etiology of pregnancy complications and pre-eclampsia.
CONCLUSION
In the present study, demonstrating gender-based changes lends support to the idea of the active contribution of the placenta to maternal metabolism during pregnancy.
LIST OF ABBREVIATIONS
| ELISA | = Enzyme Linked-Immuno–Sorbent Assay |
| FDVD | = Full-Term Vaginal Delivery |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The institutional ethical committee approved the experimental procedures and protocols (Pandit Bhagwat Dayal Sharma Post Graduate Institute of Medical Sciences).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans used were per the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
Informed consent was obtained.
STANDARDS OF REPORTING
STROBE guidelines and methodology were followed.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available within the article.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to acknowledge residents, lab technicians, and women participated in the study.


