All published articles of this journal are available on ScienceDirect.
Corrected ESR/Albumin Ratio as a Simple, Practical Marker Predicts the Severity in Egyptian Ulcerative Colitis Patients
Abstract
Background:
Serum biomarkers are commonly used for diagnosing and monitoring the disease activity of Ulcerative Colitis (UC) patients. However, their role in predicting disease severity among Egyptian patients is unknown.
Objectives:
The aim of this study was to correlate these biomarkers with clinical, endoscopic and histologic severity.
Methods:
This is a cross-sectional survey where 55 patients with UC were included to measure corrected Erythrocyte Sedimentation Rate (ESR), hematocrit (Hct), corrected ESR/albumin ratio and albumin, as well as colonoscopy and biopsy. Sensitivity and specificity, positive and negative predictive values were correlated with clinical, endoscopic, histologic severity.
Results:
The mean age of patients was 33 ± 8.4 years. In total, 27 (49.1%) were males and 28 (50.9%) were females. Area Under the Curve (AUC) values for the diagnosis of severe clinical disease were 0.947, 0.932, 0.727 and 0.685 for corrected ESR/albumin ratio, corrected ESR, Hct and albumin, respectively. Cut-off value to determine endoscopic severity for Hct was 34 (sensitivity: 88.89%, specificity: 83.78%, PPV: 72.7%, NPV: 93.9%, AUC: 0.963, p<0.001).
Conclusion:
Corrected ESR/albumin ratio was the best predictor of severe clinical activity of UC disease. Hct may be a marker of endoscopic and histological severity due to its high sensitivity and specificity as a diagnostic test.
1. INTRODUCTION
Ulcerative Colitis (UC) is a chronic inflammatory intestinal disease (IBD) characterized by a disease relapse and period of remission [1]. Abdominal pain and blood-mixed diarrhea are the principal flags of active illness [2, 3].
UC specifically targets the superficial layers of the colonic lining translated into endoscopic findings, including mucosal edema, erythema, granularity, friability and ulcers. Based on combined clinical and endoscopic evaluations, disease activity can be graded as mild, moderate, severe or fulminant [4, 5]. In addition, the endoscopic index of severity of ulcerative colitis (UCEIS) has been recently developed and validated as a method for accurately predicting the overall endoscopic severity assessment of UC [6, 7].
Mayo Endoscopic Score (MES) has been commonly used in research studies since 1987, and a grade between 0 and 1 is used as mucosal healing descriptor (MH) [8]. Nevertheless, the use of repeated endoscopy to check MH would be intrusive, painful and costly, and could be a risk of significant complications (i.e. colonic perforation). Consequently, non-invasive surrogate markers suggesting endoscopic healing were evaluated to replace repeated endoscopic procedures [9-13]. The main biomarkers in IBD are C-reactive Protein (CRP), Erythrocyte Sedimentation Rate (ESR), White Blood Cells (WBC), acid glycoprotein, platelet count, fecal and serological marker albumin [14-16]. But, for predicting IBD activity, they have a little precision [17].
Albumin is an example of a negative acute phase reactant and may be found to have diminished levels during inflammatory conditions. A chronic inflammatory condition such as UC can affect albumin levels in the body in several ways [18].
The determination of the Erythrocyte Sedimentation Rate (ESR) represents the variations in the different acute-phase proteins. ESR varies with concentrations of plasma protein and hematocrit (Hct) value [19, 20]. Although the International Council for Standardization in Hematology (ICSH) recommends Westergren as a reference method for ESR, this method has limitations with regard to low rates of hematocrits and requires more time and large quantities of specimens to test [21, 22]. As the Westergren method overestimates ESR in low Hct specimens, Fabry's formula has been presumed to correct ESR calculation [21, 23].
In view of these considerations, the current research aimed to facilitate disease activity monitoring, predict the severity in UC by noninvasive markers (a combination of corrected ESR and albumin); as well as correlate these markers to clinical, endoscopic and histological scores and severity of the disease.
2. MATERIALS AND METHODS
2.1. Study Design and Setting
A prospective cross-sectional study was conducted among 55 Ulcerative colitis patients who had been attending the Department of Hepatology, Gastroenterology and Infectious Diseases, Kafr El-Sheikh University Hospital, Egypt, between January, 2018 and January, 2020. Informed consent was taken in writing by patients who agreed to participate before enrollment in this study.
2.2. Inclusion Criteria
The diagnosis of patients suffering from UC who agreed to participate and were included in this study was based on clinical data, pathological findings and endoscopic evaluation.
2.3. Exclusion Criteria
Patients who refused to participate in the study or to undergo colonoscopy; patients with other forms of IBD such as Cohn's disease, acute infectious colitis; patients with chronic diseases of the liver, malignancy, chronic diseases of the kidney, connective tissue disease, heart failure, vacuities, celiac disease, history of chronic Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) intake or exposure to radiation were excluded from the study.
2.4. Method
Fifty-five ulcerative colitis patients were subjected to full history taking and thorough physical examination. All the patients were evaluated regarding albumin (NR, 3.5–5.5 g/dL; using the modified bromocresol green colorimetric method) [24], Hct levels (NR, 41–53%) and ESR (ESR; NR 0–15 mm/h; Westergren method). Corrected ESR for the actual Hct was calculated using Fabry’s formula: Hct-Corrected ESR= ESR x 15/ 55-hematocrit [23]. Also, corrected ESR/ serum albumin ratio was calculated for all patients.
All patients had undergone a colonoscopy within 3 days of the aforementioned laboratory tests and multiple biopsies from inflamed areas were taken and put in tubes containing a predefined volume of 4% neutral buffered formalin. A paraffin block was made and a corresponding 5-µm haematoxylin-eosin stained slide was prepared for histopathological assessment.
The clinical activity of the disease was classified according to Trulove and Witts’ criteria for UC into mild, moderate or severe [25]. Qualified gastroenterology doctors evaluated the endoscopic activity using standardized colonoscopy definitions (EG-3890, Pentax, Tokyo, Japan). Endoscopic activity was assessed using MES. According to this index, patients with the normal appearance of the endoscopic mucosal have been described as Mayo: 0; those with the existence of mild friability, mucosal erythema and reduced vascular pattern have been described as Mayo: 1(mild activity); those with the existence of friability and erosions, marked erythema and the disappearance of the vascular pattern have been described as Mayo: 2 (moderate activity) and those with the existence of spontaneous bleeding and ulceration have been described as Mayo: 3 (severe endoscopic activity) [8]. The haematoxylin and eosin stain (H&E) stained specimens were histologically classified according to Geboes Score from 0-5 grade [26].
2.5. Statistical Analysis
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). The Kolmogorov- Smirnov, Shapiro and D’agstino tests were used to verify the normality of the distribution of variables. ANOVA was used for comparing the four studied groups. Kruskal Wallis test was used to compare different groups for abnormally distributed quantitative variables. The Receiver Operating Characteristic curve (ROC) was used to determine the diagnostic performance of the markers. Area more than 50% gives acceptable performance, and area about 100% is the best performance for the test. The correlation coefficient was determined by spearman's correlation. The significance of the obtained results was judged at the 5% level.
3. RESULTS
Fifty-five patients with ulcerative colitis were enrolled in our study, 27 (49.1%) patients were female and 28 (50.9%) were male. The mean age was 33 ± 8.4years. According to the clinical activity index, 10.9% were in remission, 25.5% had mild disease, 36.4% had moderate disease and 27.3% had severe disease (Table 1).
| Parameters | No. (%) |
|---|---|
| Sex | |
| Male | 27(49.1%) |
| Female | 28(50.9%) |
| Age (years) | |
| <30 | 15 (27.3%) |
| 30 – <40 | 27 (49.1%) |
| ≥40 | 13 (23.6%) |
| Median (Min. – Max.) | 33 (15 –55) |
| Mean ± SD. | 33 ± 8.4 |
| Smoking | |
| No smoker | 44 (88%) |
| Smoker | 11 (20%) |
| Serum albumin (g/dl) | |
| Median (Min. – Max.) | 3.5 (2.9 – 5) |
| Mean ± SD. | 3.6 ± 0.5 |
| Hematocrit% | |
| Median (Min. – Max.) | 36 (27 – 48) |
| Mean ± SD. | 36.8 ± 5.5 |
| Corrected ESR/albumin ratio | |
| Median (Min. – Max.) | 6.3 (2.9 – 17.2) |
| Mean ± SD. | 7.3 ± 3 |
| Corrected ESR, mm | |
| Median (Min. – Max.) | 22.5 (12.5 – 50) |
| Mean ± SD. | 25.8 ± 8.9 |
| Clinical activity | |
| Remission | 6 (10.9%) |
| Mild | 14 (25.5%) |
| Moderate | 20 (36.4%) |
| Severe | 15 (27.3%) |
| Median (Min. – Max.) | 3 (1 – 4) |
| Mean ± SD. | 2.8 ± 1 |
| Index of severity by colonoscopy (mayo score) | |
| Mild | 17 (30.9%) |
| Moderate | 20 (36.4%) |
| Severe | 18 (32.7%) |
| Median (Min. – Max.) | 2 (1 – 3) |
| Mean ± SD. | 2 ± 0.8 |
| Geboes histologic score | |
| Median (Min. – Max.) | 3 (1 – 5) |
| Mean ± SD. | 3.1 ± 1.4 |
There was a statistically significant difference regarding corrected ESR, Hct, corrected ESR/albumin ratio and albumin levels (p<0.001). When patients were classified into categories according to their clinical activities, they were mild, moderate, severe and remission groups. As the clinical activity of the disease increased from remission to severe activity, corrected ESR and corrected ESR/albumin ratio increased significantly, while albumin values and Hct decreased significantly (p<0.001) (Table 2).
When the laboratory findings were evaluated according to endoscopic disease severity (mild, moderate and severe), a significant difference was detected between the median of three groups. As MES increased, corrected ESR (p=0.010) and corrected ESR/albumin ratio (p<0.001) showed a significant increase, while albumin and Hct showed a significant decrease (p<0.001) (Table 3).
Patients with high Geboes histologic score were found to have significantly higher corrected ESR and corrected ESR/albumin ratio; significantly lower albumin and Hct levels than those with low Geboes histologic score (p<0.001). However, there was no significant difference in corrected ESR among the groups (p=0.056), so the combined tests (corrected ESR/albumin ratio) provided better detection of histological activity of UC than corrected ESR alone (Table 4).
| - | Clinical Activity | Test of sig. | p | |||
|---|---|---|---|---|---|---|
|
Remission (n=6) |
Mild (n=14) |
Moderate (n=20) |
Severe (n=15) |
|||
| Serum albumin (g/dl) | ||||||
| Median (Min. – Max.) | 4.1(3.7–4.3) | 4.3(3.3–5) | 3.3(3–4) | 3.4(2.9–4) | F= 26.171* |
<0.001* |
| Mean ± SD. | 4.1 ± 0.2 | 4.2 ± 0.5 | 3.3 ± 0.2 | 3.4 ± 0.4 | ||
| Hematocrit% | ||||||
| Median (Min. – Max.) | 38.5(36–40) | 44(32–48) | 34(30–41) | 32(27–46) | F= 10.158* |
<0.001* |
| Mean ± SD. | 38.2 ± 2 | 42.1 ± 4.8 | 34.9 ± 3 | 33.9 ± 6.2 | ||
| Corrected ESR/ albumin ratio | ||||||
| Median (Min. – Max.) | 4.1(2.9–4.8) | 5.2(3.6–6.2) | 6.8(5.1–12.5) | 11.1(6.5–17.2) | H= 43.118* |
<0.001* |
| Mean ± SD. | 4 ± 0.6 | 5.2 ± 0.6 | 7.1 ± 1.7 | 10.8 ± 2.7 | ||
| Corrected ESR, mm | ||||||
| Median (Min. – Max.) | 16.2(12.5–19) | 22.1(15–27.9) | 21(19.1–37.5) | 35.5(22.1–50) | H= 31.773* |
<0.001* |
| Mean ± SD. | 16.3 ± 2.5 | 22 ± 3.6 | 23.3 ± 4.6 | 36.2 ± 9.1 | ||
p: p-value for comparing between the three categories
*: Statistically significant at p ≤ 0.05
| Endoscopic Mayo score | Test of sig. | p | |||
|---|---|---|---|---|---|
|
Mild (n=17) |
Moderate (n=20) |
Severe (n=18) |
|||
| Serum albumin (g/dl) | |||||
| Median (Min. – Max.) | 4.3 (3.7–5) | 3.4(3–4) | 3.2(2.9–3.5) | F= 59.873* |
<0.001* |
| Mean ± SD. | 4.3 ± 0.3 | 3.4 ± 0.3 | 3.3 ± 0.2 | ||
| Hematocrit% | |||||
| Median (Min. – Max.) | 43(36–48) | 36.5(30–46) | 32(27–36) | F= 32.513* |
<0.001* |
| Mean ± SD. | 42.1 ± 3.8 | 36.7 ± 4.5 | 31.9 ± 2.5 | ||
| Corrected ESR/albumin ratio | |||||
| Median (Min. – Max.) | 4.9(2.9–6.2) | 6.6(4.7–12.5) | 7.9(6.3–17.2) | H= 31.019* |
<0.001* |
| Mean ± SD. | 4.8 ± 0.9 | 7.8 ± 2.6 | 9.1 ± 3.1 | ||
| Corrected ESR, mm | |||||
| Median (Min. – Max.) | 21(12.5–27.9) | 22.6(18.3–50) | 25.4(20–50) | H= 9.277* |
0.010* |
| Mean ± SD. | 20.6 ± 4.5 | 26.6 ± 8.9 | 29.7 ± 9.8 | ||
p: p-value for comparing between the three categories
*: Statistically significant at p ≤ 0.05.
| - | Histological Activity (Geboes score) | Test of sig. | p | |||
|---|---|---|---|---|---|---|
|
1 (n= 9) |
2 (n= 13) |
3 (n= 9) |
Severe (n=24) |
|||
| Serum albumin (g/dl) | ||||||
| Median (Min. – Max.) | 4.3 (3.7 – 5.0) | 4.0 (3.3 – 4.5) | 3.4 (3.0 – 4.3) | 3.3 (2.9 – 4.0) | F= 18.359* |
<0.001* |
| Mean ± SD. | 4.3 ± 0.4 | 3.9 ± 0.4 | 3.6 ± 0.5 | 3.3 ± 0.3 | ||
| Hematocrit% | ||||||
| Median (Min. – Max.) | 40.0 (40.0 – 48.0) | 43.0 (38.0 – 46.0) | 36.0 (33.0 – 37.0) | 32.0 (27.0 – 37.0) | F= 56.599* |
<0.001* |
| Mean ± SD. | 42.8 ± 3.4 | 42.3 ± 3.1 | 35.4 ± 1.4 | 32.1 ± 2.6 | ||
| Corrected ESR/albumin ratio | ||||||
| Median (Min. – Max.) | 5.0 (3.6 – 6.2) | 5.9 (4.8 – 12.5) | 5.9 (2.9 – 11.1) | 7.5 (4.7 – 17.2) | H= 16.514* |
0.001* |
| Mean ± SD. | 4.9 ± 0.8 | 7.0 ± 2.3 | 6.2 ± 2.5 | 8.7 ± 3.3 | ||
| Corrected ESR, mm | ||||||
| Median (Min. – Max.) | 20.0 (15.0 – 27.9) | 24.0 (19.1 – 50.0) | 19.7 (12.5 – 35.5) | 24.5 (18.3 – 50.0) | H= 7.570 |
0.056 |
| Mean ± SD. | 21.5 ± 4.9 | 26.9 ± 8.9 | 21.2 ± 6.9 | 28.4 ± 9.7 | ||
p: p-value for comparing between the three categories
*: Statistically significant at p ≤ 0.05
As indicated in Table 5, the corrected ESR/albumin ratio exhibited a significant positive correlation with clinical activity, Mayo endoscopic score and Geboes histologic score (P<0.001), while albumin and Hct exhibited significant negative correlations correlation with previous scores (P<0.001). On the other hand, corrected ESR showed a significant positive correlation with clinical activity, MES (P<0.001, P=0.002 respectively). So, corrected ESR exhibited weaker correlations with the MES, as compared to Hct, albumin and corrected ESR/albumin ratio. Moreover, there was no correlation between corrected ESR and Geboes histologic score (P= 0.062).
In order to identify the best cut-off values that could indicate severe clinical, histological and endoscopic disease episodes, ROC analysis was conducted for the corrected ESR, Hct, albumin values and corrected ESR/albumin ratio.
Regarding the severe clinical activity of UC disease; the area under the ROC curve (AUC) for corrected ESR/albumin ratio was found to be 0.947 at cut-off point 8.2. Moreover, this cut-off point showed sensitivity (88.9%), specificity (90.3%), positive predictive value (85.1%) and negative predictive value (92.8%) (Table 6 and Fig. 1A). It is, therefore, the best predictor of severe clinical activity.
| - | Clinical activity | Mayo score | Original Geboes Score | |||
|---|---|---|---|---|---|---|
| rs | p | rs | p | rs | p | |
| Serum albumin (g/dl) | -0.616* | <0.001* | -0.751* | <0.001* | -0.673* | <0.001* |
| Hematocrit | -0.513* | <0.001* | -0.752* | <0.001* | -0.885* | <0.001* |
| Corrected ESR/albumin ratio | 0.892* | <0.001* | 0.727* | <0.001* | 0.536* | <0.001* |
| Corrected ESR | 0.715* | <0.001* | 0.413* | 0.002* | 0.254 | 0.062 |
| - | AUC | p value | 95% C.I | Cut off | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|
| L.L | U.L | ||||||||
| Serum albumin (g/dl) | |||||||||
| Clinical activity | 0.685 | 0.016* | 0.546 | 0.804 | ≤4 | 100.0 | 35.0 | 36.6 | 100.0 |
| Index of severity by colonoscopy (mayo score) | 0.812 | <0.001* | 0.683 | 0.904 | ≤3.5 | 100.0 | 62.16 | 56.2 | 100.0 |
| Histological activity (Geboes score) | 0.856 | <0.001* | 0.735 | 0.936 | ≤3.5 | 91.67 | 67.74 | 68.7 | 91.3 |
| Hematocrit | |||||||||
| Clinical activity | 0.727 | 0.016* | 0.590 | 0.838 | ≤30 | 46.67 | 97.50 | 87.5 | 83.0 |
| Index of severity by colonoscopy (mayo score) | 0.898 | <0.001* | 0.786 | 0.963 | ≤34 | 88.89 | 83.78 | 72.7 | 93.9 |
| Histological activity (Geboes score) | 0.961 | <0.001* | 0.871 | 0.995 | ≤35 | 91.67 | 93.55 | 91.7 | 93.5 |
| Corrected ESR/albumin ratio | |||||||||
| Clinical activity | 0.947 | <0.001* | 0.850 | 0.989 | >8.2 | 86.67 | 95.0 | 86.7 | 95.0 |
| Index of severity by colonoscopy (mayo score) | 0.820 | <0.001* | 0.693 | 0.910 | >6.2 | 100.0 | 72.97 | 64.3 | 100.0 |
| Histological activity (Geboes score) | 0.765 | <0.001* | 0.632 | 0.869 | >5.9 | 87.50 | 64.52 | 65.6 | 87.0 |
| Corrected ESR x15/55-hematocrit | |||||||||
| Clinical activity | 0.932 | <0.001* | 0.830 | 0.982 | >26.7 | 86.67 | 90.0 | 76.5 | 94.7 |
| Index of severity by colonoscopy (mayo score) | 0.707 | 0.003* | 0.569 | 0.822 | >19.9 | 100.0 | 35.14 | 42.9 | 100.0 |
| Histological activity (Geboes score) | 0.633 | 0.083 | 0.492 | 0.759 | >28.1 | 41.67 | 87.10 | 71.4 | 65.9 |
CI: Confidence Intervals
PPV: Positive predictive value
NPV: Negative predictive value
*: Statistically significant at p ≤ 0.05.
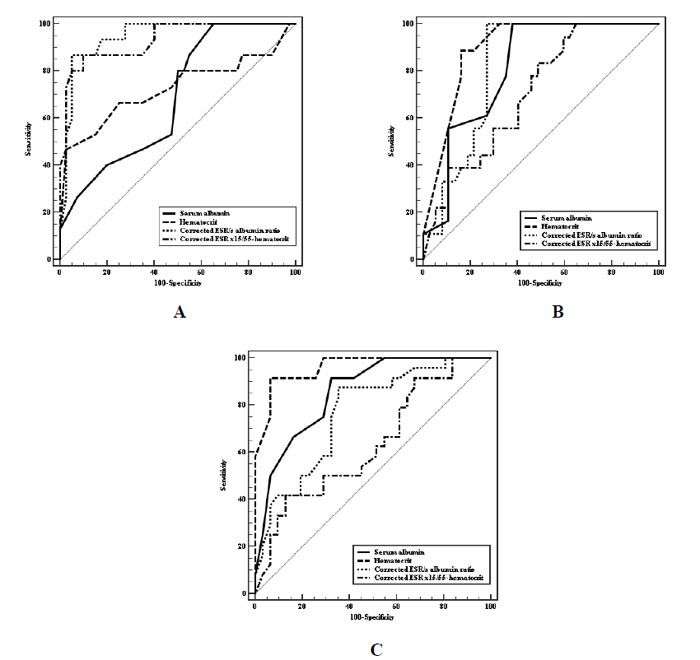
Concerning severe endoscopic activity of the disease; (AUC) for Hct was found to be 0.963 at cut-off value 34 with specificity (83.78%), sensitivity (88.89%), positive predictive value (72.7) and negative predictive value (93.9). However, (AUC) for the corrected ESR/albumin ratio was found to be 0.820 at cut-off point 6.2. In addition, sensitivity (100.0%), specificity (72.97%), positive predictive value (64.3%) and negative predictive value (100.0%) for this cut-off point were noticed at this cut-off point (Table 6 and Fig. 1B).
Severe histological activity-related (AUC) was found to be 0.961 for Hct at cut-off value 35, including sensitivity (91.67%) and specificity (93.55%). However, (AUC) for the corrected ESR/albumin ratio was found to be 0.765 at cut-off point 5.9, including sensitivity (87.50%) and specificity (64.52%) (Table 6 and Fig. 1C).
The Hct yielded the best accuracy for the determination of the severe endoscopic and histological activity of the disease at cut-off values of 34 and 35, respectively.
4. DISCUSSION
UC non-intrusive markers may have the significant advantage of avoiding invasive diagnostic tests and their complications [27]. In the diagnosis of inflammatory conditions, ESR is important [28]. ESR is an attractive alternative to new methods due to its simplicity of performance and low cost [29]. Current research findings indicate that the ESR remains high once it increases, until the primary inflammatory process is resolved [30]. The ESR procedure cannot be calibrated and is therefore vulnerable to different sources of error. There is no way to ensure multiple variables, such as hematocrit, not influencing ESR measurement [31]. In inflammatory hypoalbuminemic conditions, the ESR could also be higher than if the serum albumin was normal [32]. So in our study, we corrected ESR to overcome the hematocrit factor and correlate it with albumin. To our knowledge, this is the first study to directly compare the correlation coefficients of corrected ESR/albumin ratio with clinical activity, endoscopic severity and histological activity in comparison with corrected ESR, Hct, and albumin levels.
In the present study, the corrected ESR/s albumin ratio was the best predictor of severe clinical activity of UC disease and superior to the other laboratory parameters with a positive prediction value (r=0.892; P<0.001). Also, it was a good predictor for both endoscopic and histologic activity. However, in the current study, corrected ESR was a good predictor of severe disease episode with sensitivity and specificity 86.67% and 90.0%, respectively, which is consistent with that of Sayer et al., who reported that ESR is accurate in determining disease activity with sensitivity (88.4%) and specificity (84.6%) [33]. In our study, the corrected ESR was significantly higher in the UC patients with moderate and severe endoscopic changes, which came in accordance with the study by Hanafy et al., who reported higher ESR among these patients’ group [34]. Conversely, Minderhoud et al., found that the changes in ESR are not necessarily associated with mucosal inflammation and may not represent reliably the severity and extent of mucosal inflammation [35]. Also, Mabrouk et al., found that ESR did not adequately reflect disease activity because of low sensitivity and specificity for intestinal inflammation [36]. We found no significant correlation between corrected ESR and histological activity of the disease (p= 0.062). However, Hanafy et al., found a significantly positive correlation between ESR and clinical severity (p = 0.006, 0.71 endoscopic severity (p=0.000, 0.56) pathological activity (p=0.03, 0.32) [35].
We found statistically significant differences concerning albumin among patients with remission, low, moderate, and severe clinical activity (p<0.001). These results were in agreement with those of Sayar et al., who found the effectiveness of albumin in assessing disease severity according to clinical activity score (p= 0.001) [34]. In our study, the cut-off point for albumin in the presence of severe disease episode was 4. The sensitivity, specificity, positive predictive value, and negative predictive value for this cut-off point were 100.0%, 35.0%, 36.6%, and 100.0%, respectively. Similar results were reported by Sayar et al., who found albumin at a cut-off value of 3.6. The sensitivity, specificity, positive predictive value, and negative predictive value for this cut-off point were 91.1%, 70.8%, 66.1%, and 92.7%, respectively [34]. This negative correlation between albumin and UC disease activity could be explained by loss of protein from the intestine and malnutrition may affect serum albumin level [37]. In contrast, Ripoli et al., observed no significant difference in the levels of albumin in patients during the disease activity period compared to that of remission as they enrolled outpatient who did not require hospitalization [38]. In line with the study by Xie et al., we found a significant difference in albumin among different MES (p<0.001) [39].
In the current study, we showed that the Hct value was the best predictor of endoscopically active disease with a negative correlation (r=−0.752; P<0.001). In accordance with these results, Akpinar et al., found that Hct level had a negative correlation with the endoscopic activity of UC disease. (r=−0.318; P<0.001) [40]. Indeed, we reported that Hct was the best predictor of histologic activity of disease with a negative correlation (r=−0.885; P<0.001). These results were in agreement with those of Rodríguez et al., who found that hemoglobin (Hgb) and Hct were negative predictive values and correlated with the degree of activity in the biopsy. Within sixty patients suffering from UC, the specificity and sensitivity were (100%, 51%) for Hgb and (100%, 51%) for Hct, respectively [41]. Moreover, Reinisch et al., reported that a low level of Hgb predicted a more aggressive disease course [42]. Hgb also is useful in the early prediction of treatment response among patients with severe acute UC [43]. This can be explained by more inflammatory activity, which possibly contributes to more blood loss, increased hepcidin release and decreased iron absorption from the intestine [44]. Thus, assessing Hgb and Hct in patients in IBD is simple, fast to perform, cheap and is part of the standard of care tests [45]. However, Ripoli et al., observed no significant difference in the levels of hematocrit in patients during the disease activity period compared to that of remission [37]. Also, LOK et al., demonstrated that commonly used serum biomarkers in Chinese UC patients were positive in only a small proportion of cases with endoscopically and histologically confirmed active colonic inflammation. However, several limitations were noted in their study [46].
CONCLUSION
The current research observed that the association between corrected ESR/albumin ratio and disease clinical, endoscopic and histologic activity was significant. Hct demonstrated greater positive predictive value and specificity than either corrected ESR/albumin ratio and albumin in predicting severe endoscopic and histologic activity in ulcerative colitis. Therefore, the current study recommends that corrected ESR/albumin and Hct; cheap, simple and easily calculated parameters, could be good indicators of UC severity.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research steps were explained and the permission for conducting the research was obtained from the approving authority, Faculty of Medicine, Kafr El-Sheikh University, Egypt.
HUMAN AND ANIMAL RIGHTS
No animals were used as the basis of this study. This research was conducted according to the Declaration of Helsinki principles.
CONSENT FOR PUBLICATION
Informed consent was taken in writing by patients who agreed to participate before enrollment in this study.
AVAILABILITY OF DATA AND MATERIALS
The data sets used during the current study can be provided from the corresponding author [I.A.], upon reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We thank the members of the KFU endoscopy unit who participated and cooperated in the study.


