All published articles of this journal are available on ScienceDirect.
The Role of MicroRNAs (miRNA 155, miRNA-146b) and Procalcitonin as Novel Markers for the Diagnosis of Spontaneous Bacterial Peritonitis
Abstract
Background:
MircoRNAs are endogenous, small non-coding RNA molecules that have been recognized as important modulators of gene expression. MicroRNA is considered one of the potential biomarkers of infection and inflammation. Our study aims to identify the potential role of miRNA-155, miRNA-146b, and Procalcitonin (PCT) in the early detection of spontaneous bacterial peritonitis in cirrhotic liver patients. miRNA-155 and 146b are molecular biomarkers , while procalcitonin is a serum marker in ascites patients complicated with Spontaneous Bacterial Peritonitis (SBP) .
Methods:
This study was conducted on 199 patients, 101 of them have ascites complicated with spontaneous bacterial peritonitis, and 98 patients without spontaneous bacterial peritonitis (control group). Ascitic fluid samples were collected from patients with SBP undergoing paracentesis at National Hepatology Institute in Egypt. MicroRNAs were determined in the serum using qPCR (quantitative polymerase chain reaction), while procalcitonin has been assessed in serum samples using ELISA (Enzyme-linked immune assay) technique.
Results:
Serum levels of miRNA-146b & miRNA-155 were significantly higher (p<0.001) in spontaneous bacterial peritonitis patients (79.2% and 97.0% respectively) than ascites patients (17.3% and 7.1%, respectively). Furthermore, the serum level of procalcitonin was significantly higher (p<0.001) in spontaneous bacterial peritonitis patients than that in ascites patients (68.3% and 27.6%, respectively).
Conclusion:
miRNA-155, miRNA-146b and procalcitonin can be used as early markers for the detection of SBP in hepatic patients with ascites.
1. INTRODUCTION
1.1. Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis is defined as an ascitic fluid infection without an evident intra-abdominal, surgically treatable source [1]. The pathogenesis of spontaneous bacterial peritonitis is attributed to the altered immune response that leads to its dysfunction in decompensated liver cirrhosis disea- ses [2]. This in addition to the leaky gut syndrome theory that implies a relative vulnerability of the gut mucosa, leading to bacterial and endotoxins translocation from the bowel lumen into the ascitic fluid [3, 4]. Diagnosis of spontaneous bacterial peritonitis is made by a positive ascitic fluid bacterial culture [5]. The prevalence of spontaneous bacterial peritonitis in patients with cirrhosis ranges from 10 to 30% [6]. Spontaneous bacterial peritonitis represents about 2.5% of the causes of hospitalization in patients with liver cirrhosis [7]. A one-year mortality rate, after the first episode of spontaneous bacterial peritonitis, ranges from 31% to 93% [8]. In hospitalized patients, spontaneous bacterial peritonitis are accompanied by jaundice, encephalopathy and low blood pressure and diag- nostic paracentesis should be done immediately on admission to confirm the diagnosis [9].
1.2. MiRNAs as Biomarkers
Recent studies showed that several types of miRNAs can be used in the early detection of spontaneous bacterial peritonitis by their expression in the ascitic fluid of a cirrhotic patient. Yang et al., reported high expression levels of miR-21 and miR-186 in patients with peritoneal carcinomatosis, whereas miR-223 was significantly upregulated in patients with spontaneous bacterial peritonitis [10]. MicroRNA (miRNA) was first discovered in 1993 [11]. They were defined as small, non-coding RNAs of about 21–25 nucleotides [12]. MiRNAs regulate gene expression by binding to messenger RNA (mRNA), leading to posttranscriptional changes by targeting the 3'-UTR of mRNA to down-regulate gene expression [13]. MiRNAs are considered one of the potential biomarkers for a variety of diseases, including inflammatory liver diseases [14], because of their minute size and ability to circulate [15]. They are stable and therefore can be detected in plasma or serum, which made them an uprising potential biomarker for diagnosis and prognosis. Additionally, microRNAs control the activity of more than 50% of all protein-coding genes. MiRNA-155 was reported to be up-regulated in various types of diseases. It is one of 15 miRNA that has been reported to be expressed differentially in breast cancer. Additionally, miRNA-155 promotes the development of non-small lung cancer and is found to be commonly expressed in other types of cancer, such as pancreatic cancer [16].
1.3. Procalcitonin as a Biomarker
Procalcitonin is considered one of the significant serum biomarkers that are linked to several types of infections and inflammations [17, 18]. Procalcitonin is a calcitonin precursor 116-amino-acid peptide that is secreted from extrathyroidal cells (e.g. monocytes). Serum levels of procalcitonin in healthy individuals are undetectable by clinical assays (<0.01 ng/mL). However, in bacterial infections, serum procalcitonin levels start to rise 4h after the onset of systemic infection and peak between 8 and 24h. The half-life of procalcitonin in serum is 20 to 24h, which makes it suitable for early detection and daily monitoring of infections owing to its short half-life [18]. Several studies have investigated the diagnostic value of Procalcitonin in liver disease patients with spontaneous bacterial peritonitis. Results on the accuracy of procalcitonin in the early detection of bacterial infection in liver cirrhosis, especially spontaneous bacterial peritonitis, are controversial [19]. A study by Wu et al., 2016, performed a meta-analysis and reported moderate to high accuracy for Procalcitonin as a diagnostic aid for SBP. Since then, other studies of Procalcitonin have further increased our understanding of the reliability of procalcitonin as an SBP diagnostic marker [20]. Procalcitonin has been reported to be superior to C-reactive protein in discriminating infectious from other inflammatory diseases such as acute pancreatitis, cardiogenic shock, and acute transplant rejection [21]. Our study aims to identify the potential role of miRNA-155, miRNA-146b as a molecular marker, and procalcitonin as a serum marker in ascites patients complicated with spontaneous bacterial peritonitis.
2. MATERIALS AND METHODS
2.1. Patients & Subjects
This study was conducted on 199 total number of patients, divided into 2 groups; group I comprised 101 patients with ascites complicated with spontaneous bacterial peritonitis, group II comprised 98 non-complicated ascites patients (control group). All subjects were recruited from out-patients clinics in National Hepatology & Tropical Medicine Research Institute in Cairo.
2.2 Ethical Approval
All participants were subjected to detailed medical history records investigation and full clinical examination. Informed consent was taken for each participant in this study, the content was reviewed by the ethical committee of the faculty of pharmacy Suez canal university (201909MH1).
2.3. Diagnosis
Diagnosis of liver cirrhosis, ascites, and SBP was done using general clinical examination, local abdomen examination, and confirmed by abdominal ultrasound and ascitic fluid analysis.
2.4. Inclusion and Exclusion Criteria
- Inclusion criteria include patients with an Age range from 19 to 77 years old, with a body mass index of ≤32. Ascetic patients without spontaneous bacterial peritonitis were used as a control group, and ascetic patients with spontaneous bacterial peritonitis (investigation group).
- Exclusion criteria were as follows; patients with eviden- ce of gastrointestinal bleeding or bacterial infection in the preceding 6 weeks, treatment with a non-absorbable antibiotic in the preceding 6 weeks before the start of the study in addition to other non-peritoneal infection (skin infection, chest infection, biliary tract infection, urinary tract infection, gastroenteritis, dental infection, and meningitis).
2.5. Laboratory Parameters
Standard laboratory parameters (serum albumin, bilirubin, creatinine, C-reactive protein, (international normalized ratio), sodium, total blood count, ascites total protein & ascites leukocyte) were measured. The following biochemical tests were done for all involved subjects: Aspartate aminotransferase, alanine aminotransferase, creatinine, total bilirubin, and albumin were assayed using OLYMPUS automatic analyzer AU400.
Our study aims to determine Procalcitonin level by Enzyme-linked immune assay & miRNA-155 and miRNA-146b by (Reverse transcriptase-polymerase chain reaction). The severity of liver disease was assessed by modified Child-Pugh score [22], model for the end-stage liver disease [23], and updated MELD score [24].
2.6. Sampling and Isolation of MiRNA
-
1- Extraction and purification of 155, 146b miRNAs: Plasma or serum samples were thawed and prepared.
- 500 μl of QIAzol Lysis Reagent were added to each sample and mixed with vortexed or by pipetting up and down.
- Tubes that contain homogenate were placed on a benchtop at room temperature (15-25°C) for 5min.
- 100 μl of chloroform were added to each homogenate tube and closed securely and vortexed vigorously for 15 s.
- Tubes that contain homogenate were placed on a benchtop at room temperature (15-25°C) for 2-3 minutes.
- Each tube was centrifuged for 15 min at 12,000 x g at 4°C.
- (After centrifugation, the sample was separated into 3 phases: an upper, colorless, aqueous phase containing RNA [300 μl]; a while interphase; and a lower, red, organic phase.)
- The upper aqueous phase of each tube [300 μl] was transferred to a new collection tube, 450 μl of 100% ethanol were added to each collection tube and mixed thoroughly by pipetting up and down several times, without a centrifuge.
- 700 μl of the sample, including any precipitate that may have formed, was pipetted up into an RNeasy Mini spin column in a 2 ml collection tube (both supplied). The lid was closed gently and centrifuged at ≥8000 x g (≥10,000 rpm) for 15 s at room temperature (15–25°C). The flow-through was discarded.
- In the same collecting tube, the last step was repeated using the remainder of the sample.
- In the same collecting tube, 700 μl Buffer RWT was added to the RNeasy Mini spin column. The lid was closed gently and centrifuged for 15 s at ≥8000 x g (≥10,000 rpm) to wash the column. The flow-through was discarded.
- In 500 μl Buffer, RPE was added onto the RNeasy Mini spin column. The lid was closed gently and centrifuged for 15 s at ≥8000 x g (≥10,000 rpm) to wash the column. The flow-through was discarded.
- The last step was repeated using the same collecting tube.
- The old collection tube with the flow-through was discarded and the RNeasy Mini spin column was placed into a new 2 ml collection tube then Centrifuged in a microcentrifuge at full speed for 2 min.
- RNeasy Mini spin column was transferred to a new 1.5 ml collection tube, 30–50 μl RNase-free water was pipetted directly onto the RNeasy Mini spin column membrane, The lid closed gently and centrifuged for 1 min at ≥8000 x g (≥10,000 rpm) to elute the RNA.
- 2- All samples were collected and stored at -80°C.
2.7. Statistical Analysis
Data were analyzed using IBM SPSS advanced statistics (Statistical Package for Social Sciences), version 24 (SPSS Inc., Chicago, IL). Descriptive statistics were presented as median and range or mean and standard deviation for continuous variables and number and percentage for categorical variables. Chi-square (Fisher's exact) test was used to examine the relation between qualitative variables when appropriate. Multivariate analysis was done for variables statistically significant on a univariate level to indicate independent prognostic factors and to obviate the effect of confounders using a logistic regression model. Receiver Operating Characteristics curve was done to estimate the best cut off point then a calculation of sensitivity, specificity with their 95% confidence interval was done. Bonferonni corrections of p-value were done to avoid hyperinflation of type 1 error which arises from multiple testing. A p-value less than or equal to 0.05 was considered statistically significant. All tests were two-tailed.
3. RESULTS
The demographic and biochemical data of the studied groups are summarized in Table 1. This study was conducted on 199 patients, divided into 2 groups, group I comprised 101 patients with ascites complicated with spontaneous bacterial peritonitis, group II is comprised 98 patients with ascites only not complicated with spontaneous bacterial peritonitis (control group). 45.5% of patients with spontaneous bacterial peritonitis are above 37 years old and 54.5% of them below 37 years old, while 55.1% of patients with non-complicated ascites are above 37 years old and 44.9% of them are below 37 years old. There was a male predominance in both groups where they represented 52.6% of spontaneous bacterial peritonitis patients and 60% of ascites patients, with no statistically significant difference between the 2 patients’ groups. Most spontaneous bacterial peritonitis patients, as well as ascites patients, were child grade C (67.8% and 70.4%, respectively). Model for end-stage liver disease score was higher in spontaneous bacterial peritonitis patients (9.33 ± 2.79) than ascites patients (8.65 ± 2.03), yet this was not statistically significant.
On the other hand, the mean values of the updated model for end-stage liver disease were significantly higher in the spontaneous bacterial peritonitis patient group than in the ascites patient group (10.31 ± 2.68 and 8.95 ± 0.99, respectively). Alanine transaminase, aspartate aminotrans- ferase, alpha-fetoprotein, Serum creatinine, international normalized ratio, gamma-glutamyl transferase, total leucocytic count, and body mass index were significantly higher in spontaneous bacterial peritonitis patients than ascites patients (p<0.001). Platelet count and serum albumin were significantly lower in spontaneous bacterial peritonitis patients that ascites patients (p<0.001).
Table 2 shows the ascitic fluid analysis of studied groups, where the mean value of total leucocyte count, polymorphs, and protein were significantly higher in spontaneous bacterial peritonitis patients group than ascites patients group (p<0.001). Nonetheless, the mean value of serum ascitic albumin gradient was significantly lower in the spontaneous bacterial peritonitis patients group than in the ascites patients group (p<0.001). Moreover, ascitic fluid glucose level was lower in spontaneous bacterial peritonitis patients than ascites patients; however, not statistically significant (p=0.866). On the other hand, ascetic fluid albumin was higher in spontaneous bacterial peritonitis patients than ascites patients without any statistical significance (p= 0.183).
Laboratory parameters that significantly predicted the presence of spontaneous bacterial peritonitis were alanine transaminase (Odd ratio= 5.773, p<0.001), body mass index (Odd ratio =20.758, p<0.001), gamma-glutamyl transferase (Odd ratio = 20.027, p<0.001), and procalcitonin (Odd ratio = 13.478, p<0.001) (Table 3).
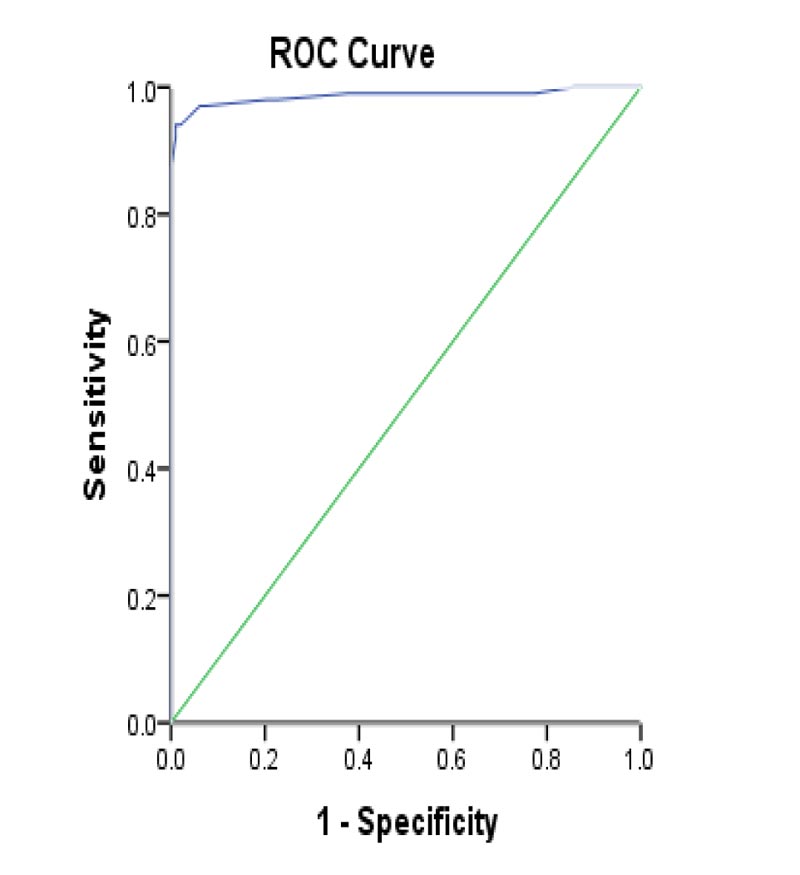
The serum levels of miRNA-146b & miRNA-155 were significantly higher (p<0.001) in spontaneous bacterial peritonitis patients than in ascites patients (79.2% and 97.0%, respectively). We used miRNA-146b 0.02275 ng/mL as the optimal cutoff value to diagnose spontaneous bacterial peritonitis, which has a sensitivity and specificity of 79.2% and 82.7%, respectively. The area under the curve was 0.834 (95% Confidence Interval: 0.774-0.894). Furthermore, miRNA-155 was with an optimal cutoff value of 0.02310 ng/ml with sensitivity and specificity of 97.0% and 93.0%. The area under the curve was 0.986 (95% Confidence Interval 0.969-1.000). Data are shown in Tables 1, 4, and 5 and Figs. (1 and 2).
|
- |
SBP Patients (n=101) |
Non SBP Patients (n=98) |
P value |
|---|---|---|---|
|
Age (mean± SD) |
39.31 ± 9.15 |
40.85 ± 15.36 |
0.399 |
|
Gender Male Female |
- 51 (52.6%) 46 (47.4%) |
- 54 (60%) 36 (40%) |
- - 0.307 |
|
Aetiology HCV positive HBV positive |
- 65 (64.6%) 36(35.4%) |
- 55 (56.2%) 43 (43.8%) |
- - 0.372 |
|
Child Pugh grade B C |
- 35 (34.7%) 66 (67.8) - |
- 29 (29.6%) 69 (70.4%) - |
- - 0.445 |
|
MELD Score (mean ± SD) |
9.33 ± 2.79 |
8.65 ± 2.03 |
0.059 |
|
uMELD Score: (mean± SD) |
10.31 ± 2.68 |
8.95 ± 0.99 |
<0.001 |
|
ALT |
40.30 ± 19.45 |
30.06 ± 5.52 |
<0.001 |
|
AST |
43.63 ± 22.21 |
32.52 ± 6.95 |
<0.001 |
|
T.Bil |
1.49 ± 1.18 |
0.76 ± 0.19 |
<0.001 |
|
S. Creatinine (mean± SD) |
1.6 ± 0.48 |
0.95 ± 0.16 |
<0.001 |
|
INR (mean± SD) |
1.25 ± 0.24 |
0.98 ± 0.07 |
<0.001 |
|
S. Albumin |
2.26 ± 0.66 |
2.86 ± 0.21 |
<0.001 |
|
White blood cells (mean ± SD) |
- 10006 ± 3199.79 |
- 7182.65 ± 1964.06 |
- <0.001 |
|
Hb |
11.86 ± 1.97 |
11.98 ± 1.47 |
0.637 |
|
AFP |
38.31 ± 93.09 |
5.70 ± 1.68 |
<0.001 |
|
BMI |
26.02 ± 2.20 |
22.35 ± 3.55 |
<0.001 |
|
GGT |
62.05 ± 39.41 |
35.81 ± 9.67 |
<0.001 |
|
Procalcitonin (mean ± SD) |
1.44 ± 0.86 |
0.65 ± 0.57 |
<0.001 |
|
Micro RNA 155 (mean±SD) |
- 0.2108 ± 0.0895 - |
0.0188 ± 0.0070 |
<0.001 |
|
Micro RNA 146 (mean±SD) |
0.0229 ± 0.0046 |
0.0193 ± 0.0063 |
<0.001 |
| Ascitic Fluid Analysis | SBP patients (n=97) |
Non SBP patients (n=90) |
P value |
|---|---|---|---|
| Total leucocyte count (mean ± SD) |
- 3428 ± 3234.8 |
- 878.6 ± 2098.7 |
- <0.001 |
| Polymorphs (mean ± SD) |
- 705.09 ± 606.29 |
- 91.84 ± 23.96 |
- 0.008 |
| Ascitic Albumin (mean ± SD) |
- 0.64 ± 0.32 |
- 0.58 ± 00.22 |
- 0.183 |
| Protein (mean ± SD) |
2.08 ± 0.45 - |
- 2.40 ± 0.96 |
- <0.001 |
| Ascitic glucose (mean ± SD) |
48.96±6.19 - |
- 81.61 ± 67.03 |
- 0.866 |
| Serum ascitic albumin gradient (mean ± SD) |
- 2.27 ± 0.86 |
- 2.64 ± 3.01 |
- <0.001 |
| - | Beta Coefficient | Standard Error | p value | OR | 95% C.I. for OR | - |
|---|---|---|---|---|---|---|
| - | - | - | - | - | Lower | Upper |
| ALT | 1.753 | .477 | <0.001 | 5.773 | 2.267 | 14.699 |
| BMI | 3.033 | .524 | <0.001 | 20.758 | 7.429 | 58.007 |
| GGT | 2.997 | .520 | <0.001 | 20.027 | 7.234 | 55.440 |
| Procalcitonin | 2.601 | .497 | <0.001 | 13.478 | 5.091 | 35.683 |
| Micro RNA-146 cut-off point |
Sensitivity | Specificity | Positive Predictive Value |
Negative Predictive Value |
Total Accuracy |
|---|---|---|---|---|---|
| 0.02275 | 79.2% | 82.7% | 82.4% | 79.4 % | 80.9% |
| Area Under the Curve Area Under the Curve | Standard error | P value | 95% Confidence Interval | ||
| Lower Bound | Upper Bound | ||||
| 0.834 | 0.031 | <0.001 | 0.774 | 0.894 | |
| Micro RNA-155 cut-off point | Sensitivity | Specificity | Positive Predictive Value |
Negative Predictive Value |
Total Accuracy |
|---|---|---|---|---|---|
| 0.02310 | 97.0% | 93.0 % | 93.3.2% | 96.8 % | 95.0% |
| Area Under the Curve | Standard error | P value | Asymptotic 95% Confidence Interval | ||
| Lower Bound | Upper Bound | ||||
| 0.986 | 0.009 | <0.001 | 0.969 | 1.000 | |
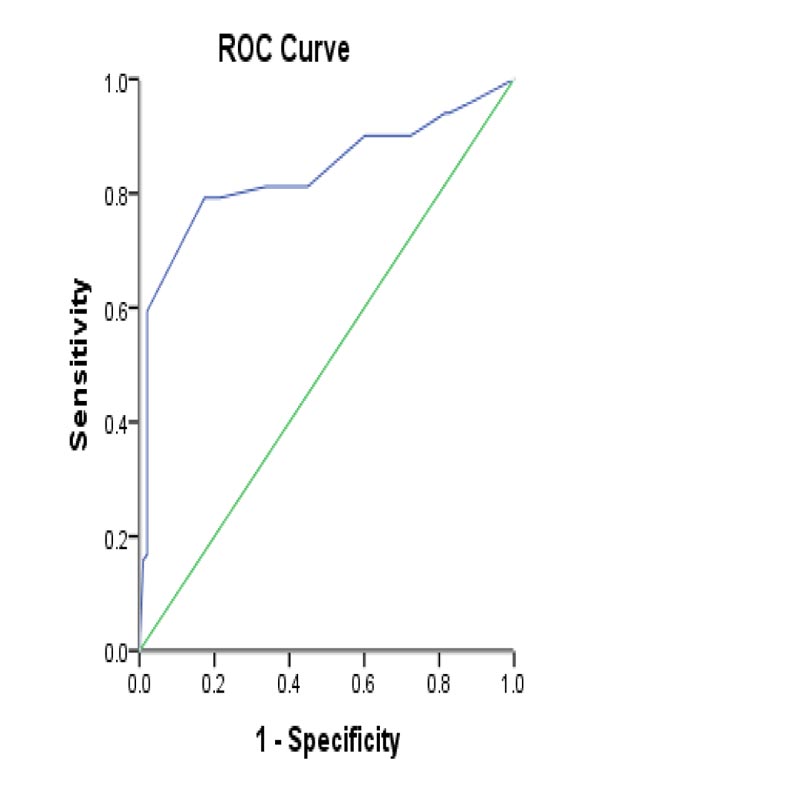
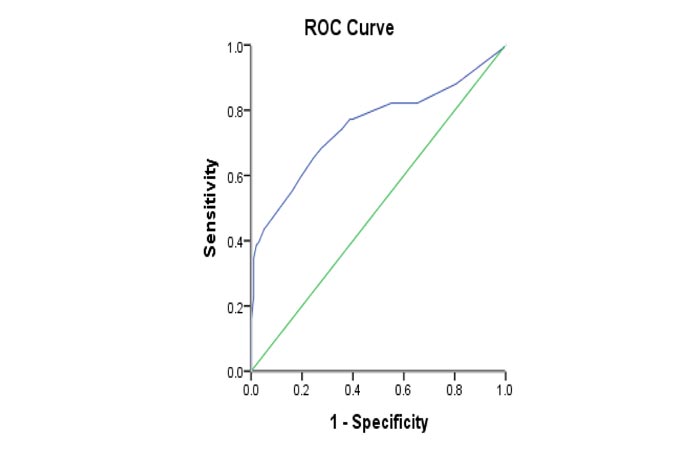
The serum level of Procalcitonin was significantly higher (p<0.001) in spontaneous bacterial peritonitis patients than that in ascites patients (68.3% and 27.6%, respectively). We used PCT 0.95 ng/mL as the optimal cutoff value to diagnose spontaneous bacterial peritonitis with sensitivity and specificity of 68.3% and 72.4%. The area under the curve was 0.751 (95% confidence interval: 0.682-0.820) (Table 6) & (Fig. 3).
4. DISCUSSION
Spontaneous bacterial peritonitis is a frequent and severe complication of cirrhotic liver patients with ascites [25]. It is a result of the accumulation of free fluid in the abdomen (peritoneal cavity) and is a feature of liver decompensation. Around 20% of people with cirrhosis have ascites [26]. This is because of the defects in the hepatic reticuloendothelial system and the peripheral destruction of bacteria by neutrophils, with secondary seeding of an ascitic fluid deficient in antibacterial activity. Patients with advanced liver disease and low ascitic fluid protein concentrations seem to have an increased susceptibility to spontaneous bacterial peritonitis [27]. Historically, gram-negative bacteria were the most frequent etiologic agents of spontaneous bacterial peritonitis, with Escherichia coli and Klebsiella species being the most frequently isolated bacteria [28].
Many studies agree on the usage of miRNAs as a diagnostic marker for many diseases because of their ability to upregulate or downregulate some type of genes. Additionally, according to the previous investigations of MiRNA-155, miRNA-155 can be used as an early diagnostic marker in a variety of diseases [ 16 ]. The approach of this study is to determine its level in spontaneous bacterial peritonitis patient’s serum and its effectiveness in combination with miRNA-146b and procalcitonin. In this case-control study, we evaluated the potential use of plasma miR-146b and miR-155 levels to predict the development of spontaneous bacterial peritonitis as one of the common complications of chronic liver disease.
MiRNAs are involved in the regulation of innate immune responses, including the development and differentiation of B and T cells, proliferation of monocytes and neutrophils, antibody switching, and the release of inflammatory mediators [29]. Ouyang et al., found that the expression of miRNA in the plasma is more significant than that in liver tissues [30]. They examined the expression of miR-146a and miR-155 in the plasma, peripheral blood mononuclear cell, and liver tissues from 41 chronic hepatitis B patients who underwent nucleoside analogs antiviral therapy for 104 weeks. Correlations between the levels of miR-146a and miR-155 among the 3 samples were analyzed. Their results showed that miR-146a and miR-155 from plasma are better expressed than those in the liver tissues. In the present study, serum levels of miRNA 155 &146b were found to be significantly higher in spontaneous bacterial peritonitis patients than that in ascites patients not complicated by spontaneous bacterial peritonitis infection. This goes in agreement with the findings of a study by Lutz et al., 2017, who found that miR-155 was significantly upregulated during spontaneous bacterial peritonitis when compared to ascites patients without spontaneous bacterial peritonitis infection [14].
| Procalcitonin cut-off point |
Sensitivity | Specificity | Positive Predictive Value |
Negative Predictive Value |
Total Accuracy |
|---|---|---|---|---|---|
| 0.95 | 68.3% | 72.4% | 71.8% | 68.9% | 70.3 |
| Area Under the Curve | Standard error | P value | 95% Confidence Interval | ||
| Lower Bound | Upper Bound | ||||
| 0.751 | 0.035 | <0.001 | 0.682 | 0.820 | |
Moreover, Jiménez-Alesanco et al. 2019, initiated a study aimed to specify the different types of exosomes released by the pancreas to the pancreatitis-associated ascitic fluid as well as those circulating in plasma in an experimental model of acute pancreatitis in rats. They reported that two different populations of exosomes are generated with relevant differences in cell distribution, protein, and microRNA. Additionally, they also found plasma exosomes, but not pancreatitis-associated ascitic fluid exosomes, are enriched in the inflammatory miR-155 and show low levels of miR-21 and miR-122. However, they found pancreatitis-associated ascitic fluid exosomes contain 10-30 fold higher loading of histones and ribosomal proteins compared to plasma exosomes. In conclusion, they report that plasma exosomes have higher pro-inflammatory activity on macrophages than pancreatitis-associated ascitic fluid exosomes [31]. In another study conducted by Nabiel et al., on the combination of CD64, calprotectin, and microRNA-155 levels as diagnostic markers of spontaneous bacterial peritonitis. They evaluated the level of MiRNA-155 in decompensated cirrhotic non-spontaneous bacterial peritonitis patients (Control group) and spontaneous bacterial peritonitis patients and approved that miR-155 levels were higher in the study group than in the control group. Their P-value was less than 0.0001 and Ascetic fluid miR-155 showed a sensitivity and specificity of 95.3 and 97.4%, respectively, at a cut-off value of 1.9. They found that the combination of the previous three markers is better and has greater efficacy in diagnosis [32].
In our study, serum procalcitonin level was slightly elevated above its cutoff value in a patient with spontaneous bacterial peritonitis with sensitivity of 68.3% and specificity of 72.4%, (with positive predictive value 71.8, negative predictive value 68.9). Many studies tackled this point and most of them were in agreement with our results. Yang et al., demonstrated that procalcitonin was more accurate than C-reactive protein for the diagnosis of bacterial peritonitis. The pooled sensitivity and specificity of serum procalcitonin for the diagnosis of bacterial peritonitis were 0.83 (95% CI: 0.76–0.89) and 0.92 (95% CI: 0.87–0.96), respectively. The positive likelihood ratio was 11.06 (95% CI: 6.31–19.38), negative likelihood ratio was 0.18 (95% CI: 0.12–0.27) and the diagnostic odds ratio was 61.52 (95% CI: 27.58–137.21) [33]. Additionally, Wang et al., suggested that composite markers of combining procalcitonin, the difference in hemoglobin concentration between newly formed and mature red blood cells, and mean fluorescence intensity of mature neutrophils could be a valuable diagnostic score to early diagnose ascites infection in patients with cirrhosis [34]. Asadi Gharabaghi et al., showed a significant association between blood procalcitonin and spontaneous bacterial peritonitis diagnosis (p=0.001). A considerable proportion of cirrhotic patients with established spontaneous bacterial peritonitis (75%) had serum procalcitonin of ≥ 0.5 ng/mL. Interestingly, they reported that although blood procalcitonin be used in rapid recognition of spontaneous bacterial peritonitis with high sensitivity and specificity, the diagnostic accuracy of this marker in the prediction of spontaneous bacterial peritonitis may decrease in patients with hepatic encephalopathy or hepatorenal syndrome [35]. Yang et al., evaluated the diagnostic role of serum procalcitonin concentrations in patients with spontaneous bacterial peritonitis, where their results showed sensitivity 0.82 (95% C.I 0.79–0.87), specificity 0.86 (95% C.I 0.82–0.89), positive likelihood ratio 4.94 (95% C.I 2.28–10.70), negative likelihood ratio 0.22 (95% C.I 0.10–0.52), and diagnostic odds ratio 22.55 (95% C.I 7.01–108.30). The area under the curve was 0.92 [19, 36]. Wu et al., found that serum procalcitonin levels in advanced liver cirrhotic patients with spontaneous bacterial peritonitis were significantly higher than those with (culture-negative neurolytic ascites). They used procalcitonin 0.78 ng/mL as the optimal cutoff value to diagnose spontaneous bacterial peritonitis, for which the sensitivity and specificity were 77.5% and 60.4%. The area under the curve was 0.706 (95% confidence interval: 0.576-0.798). The procalcitonin level was significantly correlated with the (ascitic fluid) white blood cell count (rs=0.404, P<0.01) [20, 37]. Cekin et al., reported higher procalcitonin serum levels in patients with positive bacterial culture in ascitic fluid compared to patients without culture positivity [21, 38]. Finally, Abdel-Razik et al., demonstrated that at a cutoff value of 0.94 ng/mL, serum procalcitonin had 94.3% sensitivity and 91.8% specificity for detecting spontaneous bacterial peritonitis [39].
CONCLUSION
Our study of 101 patients against control provides evidence of the ability to use miRNA-155, miRNA-146b & procalcitonin as early markers for detection of spontaneous bacterial peritonitis in hepatic patients with ascites. However, according to our results, miRNA-155, miRNA-146b levels in patients' serum are higher than procalcitonin level. Therefore, miRNA-155, miRNA-146b levels could be more significant and specific than that of procalcitonin for the detection of spontaneous bacterial peritonitis. Nonetheless, we suggest the combination of the three markers to get more specificity and accuracy for early detection of the prognosis.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has been approved by the Institutional Review Board of the Ethical Committee of the Faculty of Pharmacy, Suez Canal University, Egypt with aproval number 201909MH1.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was taken for each participant in this study.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available from corresponding author [S.A] upon reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We acknowledge the effort of Mohamed Badawy, Neurobiology and behavior department, Stony Brook University for his help in proofreading, and editing.


