All published articles of this journal are available on ScienceDirect.
Insulin Growth Factor-1 as a Predictor for the Progression of Hepatic Disease in Chronic Hepatitis B Virus Infection
Abstract
Background & Aims:
The aim of this study was to assess IGF-1 in chronic liver diseases associated with HBV infection and describe the impact of liver status on IGF-1 variables.
Methods:
This cohort study included 348 subjects and conducted between December 2018 and December 2019 at El-Sahel Teaching Hospital, Cairo, Egypt. Subjects were divided into 4 groups: group I included HBV positive hepatocellular carcinoma patients “HCC” (n= 87), group II included HBV positive patients with liver cirrhosis “LC” (n = 87), group III included chronic hepatitis B (CHB) patients with neither HCC nor cirrhosis “CHB” (n = 87) and group IV of healthy volunteers as controls (n = 87). Serum IGF-1 was measured quantitatively using a commercially available enzyme immunoassay.
Results:
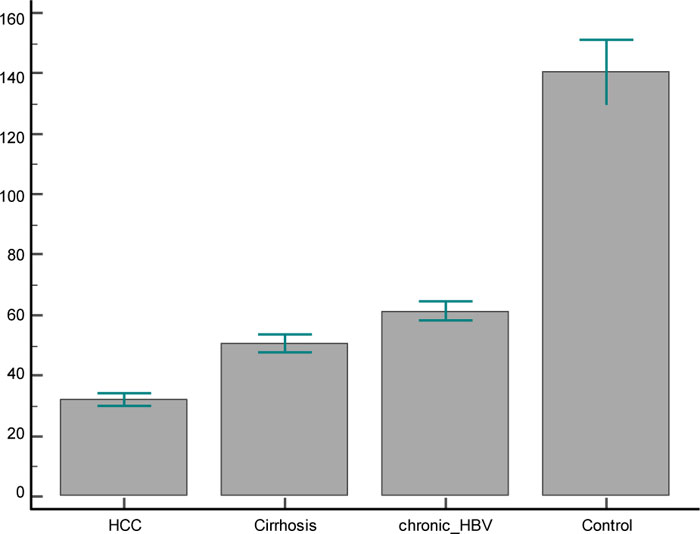
Serum levels of IGF-1 were measured in each of the 4 groups. The comparison showed marked differences in IGF1-related measures. It was found to be significantly reduced in HCC patients (32.08 ± 9.2 ng/ml), LC patients (50.6±14.1ng/ml) and CHB patients (61.4±14.3 ng/ml) in comparison to healthy subjects (140.4±49.9 ng/ml). The reduction of IGF-1 levels was also statistically significant between both HCC and LC patients and CHB patients also between HCC and LC patients.
Conclusion:
Serum IGF-1 levels are significantly reduced with the progression of hepatic disease in HBV patients and it may be a promising serological marker alone or in association with others for prediction of development of liver cirrhosis and HCC in chronic HBV patients.
1. INTRODUCTION
Hepatitis B is a global health problem, especially in the Western Pacific Region and the African Region, where 6.2% and 6.1% of the adult population is infected, respectively [1]. Clinical presentation of HBV varies from asymptomatic to fulminant hepatitis and liver failure with the risk of developing chronic hepatitis B that ranges from an asymptomatic carrier state to the signs and symptoms of cirrhosis and hepatocellular carcinoma (HCC) [2].
Globally, it is estimated that 257 million people have chronic HBV infection, resulting in about 887,000 deaths per year [3]. Based on the prevalence of HBV chronic carriers in the general population, identified by HBsAg seropositivity, the Eastern Mediterranean region is of lower-intermediate endemicity (2.00–4.99%) while HBsAg seroprevalence in Egypt is 1.71% [4]. In Egypt, HCC etiologies are variable with HCV and HBV infections, accounting for 63% and 13% of cases, respectively [5]. Globally, HBV accounts for 88% of cirrhosis-related HCC [6].
Insulin-like growth factor type 1 (IGF1) is a single-chain polypeptide hormone like insulin in structure and is important for cell proliferation and differentiation as it mediates the growth-promoting effect of growth hormone (GH) [7]. It is synthesized in the liver and secreted into the blood to act in an endocrine manner. It is also synthesized in peripheral tissues where it acts in an autocrine and paracrine manner. IGF1 synthesis by the liver and tissues is controlled by GH as well as by local tissue factors [8]. The growth hormone binds to its receptors in the liver and different tissues resulting in the upregulation of IGF1 synthesis through stimulation of IGF-I gene transcription [9]. Most IGFs in both the circulation and in local tissues are found bound to an IGF binding protein (IGFBP) that regulates IGF action through blocking its binding to its cellular receptors (IGFRs) [10]. There are six types of IGFBPs (IGFBP-1 – 6), the most predominant one that binds to about 80% of IGFs is IGFBP3, while the other IGFBPs bind to only 20% of IGFs [11].
The role of IGF system components in chronic liver diseases has been precisely investigated for better recognition and understanding of its role in liver diseases and for better management [12]. The observed deterioration of liver health associated with the decrease in the GH-IGF1 axis and the marked improvement observed when using IGF-I treatment in animal models suggest that IGF-I may be applicable for the treatment [13]. IGF-1 was also assessed as a noninvasive prognostic biomarker in patients treated with liver transplantation and the results were promising [14]. The serum level of IGF1 has also been investigated as a prognostic marker for several cancers [15-17].
Many clinical studies used serum IGF1 level to assess the hepatic function, where it revealed a reduced level of circulating IGF-I in chronic liver diseases, confirming a correlation between the serum levels of IGF and the extent of hepatocellular function [18-23]. Most of these studies were conducted on patients with chronic liver disease caused by various etiologies with those conducted in Egypt including HCV infected patients. The aim of this study was to assess IGF-1 in chronic liver diseases associated with HBV infection and describe the impact of liver status on IGF-1 variables.
2. MATERIALS AND METHODS
2.1. The Setting, Study Design
This cohort study included 348 subjects and was conducted between December 2018 and December 2019 at El-Sahel Teaching Hospital, Cairo, Egypt. Subjects were divided into 4 groups: group I included HBV positive hepatocellular carcinoma patients “HCC” (n= 87), group II included HBV positive patients with liver cirrhosis “LC” (n = 87), group III included chronic hepatitis B patients with neither HCC nor cirrhosis “CHB” (n = 87) and group IV included healthy volunteers as controls (n = 87).
Study exclusion criteria were previous cancer, current cancer apart from HCC cancer, history of alcohol abuse, renal insufficiency, proteinuria, suspected infections, clinically overt diabetes mellitus, thyroid dysfunction, or any other endocrine disorder, hormone or thyroid regulatory medication intake. Informed consent was obtained from all participants before the study. All participants gave their written informed consent prior to inclusion. The study was approved by the local ethics committee.
For all subjects, complete medical history and physical examination were done, and body mass index (BMI) was calculated. All patients were positive for HBV, confirmed by hepatitis B surface antigen (HBsAg) (Murex HBsAg, Diasorin, Saluggia, Vercelli, Italy) and HBV PCR (DNA was extracted using QIAamp DNA Blood Mini kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. The extracted DNA was amplified by Real-Time PCR using Mx 3000 (Applied Biosystems, USA)). All subjects were confirmed as HCV negative by screening for Anti-HCV using a commercially available immunoassay (HCV AB Murex, Diasorin, Saluggia, Vercelli, Italy).
2.2. Blood Sampling and Biochemical Assays
Venous blood samples (10 ml) were collected. A portion of blood was allowed to clot and then centrifuged at 3500g for 5 min to separate the serum used for assessment of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, albumin, and alfa-fetoprotein (AFP). Serum aliquots were stored at -80o C until assayed and thawed immediately before the measurement of IGF-1. Another portion of blood was collected in vacutainer tubes containing EDTA for a complete blood picture. AST, ALT, total bilirubin, albumin were assayed using OLYMPUS AU 400 chemistry analyzer (USA). The serum level of AFP was measured using CanAg AFP EIA 600-10 according to the manufacturer’s instructions. Serum IGF-1 was measured quantitatively using commercially available enzyme immunoassay DRG® IGF-1 600 ELISA (EIA-4140) Eliza, USA.
2.3. Statistical Analysis
The collected data were tabulated and analyzed using SPSS version 21 software (SPSS Inc, Chicago, ILL Company). Categorical data were presented as numbers and percentages, while quantitative data were expressed as mean ±standard deviation. Chi-square (X2) test, student “t”, Pearson correlation & one-way ANOVA tests were used as tests of significance. Significant ANOVA was followed by the Bonferroni test to detect significant pairs. The receiver operating characteristic curve (ROC) was applied to generate the cut-off value, Area under the curve (AUC), sensitivity, and specificity. The accepted level of significance in this work was stated at 0.05 (P <0.05 was considered significant).
3. RESULTS
3.1. Subjects
The clinical characteristics of the included subjects are shown in Table 1.
3.2. Serum IGF-1 Level in Patients and Control Group
In the present study, serum levels of IGF-1 were measured in each of the 4 groups. The comparison showed marked differences in IGF1-related measures (Table 2). It was found to be significantly reduced in HCC patients (32.08 ± 9.2 ng/ml), LC patients (50.6±14.1ng/ml) and CHB patients (61.4±14.3 ng/ml) in comparison to healthy subjects (140.4±49.9 ng/ml). The reduction of IGF-1 levels was also statistically significant between both HCC and LC patients and CH-B patients, and also between HCC and LC patients (Fig. 1).
| - |
Group I HCC |
Group II LC |
Group III CHB |
Group IV Healthy Control |
|---|---|---|---|---|
| No. of patients | 87 | 87 | 87 | 87 |
|
Sex Males/ females |
56/31 | 49/38 | 58/29 | 54/33 |
| Age (years) | 61.17±9.4 | 55.8±10 | 38.3±8.5 | 43.2±15.5† |
| BMI | 26.06±2.2† | 30.7±2.2 | 30.3±4.7 | 22.5±3.6† |
| INR (units) | 1.5±0.3034 | 1.5±0.8 | 1.1±0.3‡ | 0.98±0.07‡ |
| Albumin g/dL | 2.614±0.5 | 2.5±0.8 | 3.6±0.6‡ | 3.9±0.2‡ |
| Bilirubin (mg/dl) | 4.5±4.9 | 4.9±5.8 | 1.1±0.4‡ | 0.76±0.2‡ |
| ALT (units/l) | 63.08± 33.8 | 53.6±57.9 | 79.4±29.9‡ | 30.3±5.6† |
| AST (units/l) | 103.4±65 | 81.8±119.6 | 67.4±30.9* | 33.2±7.6† |
| Creatinine(mg/dL) | 2.15±1.7 | 1.6±1.2 | 1.023±0.3713‡ | 0.9±0.16‡ |
| MELD score | 20.4±9 | 18.1±9.3 | - | - |
| U MELD score | 4.140±1 | 3.9±1.3 | - | - |
|
Variable Mean + SD |
Group I HCC |
Group II LC |
Group III CHB |
Group IV Healthy Control |
|---|---|---|---|---|
| IGF-1 (ng/ml) | 32.08 ± 9.2 | 50.6±14.1* | 61.4±14.3 † | 140.4±49.9‡ |
3.3. Correlation between Serum IGF-1 Level and Patients’ Characteristics
In patients suffering from HBV infection, no significant correlations were observed between serum IGF-1 level and either the age or different biochemical findings. As we suggested, serum levels of IGF-1 as markers for progression of hepatic dysfunction, we studied that there is a correlation between serum IGF-1 level and AFP as an important marker for HCC. Nevertheless, no significant correlation was found between the levels of IGF-1 and AFP in the studied groups. Similarly, no significant correlation was detected between the IGF-1 level and the extent of cirrhosis (MELD, U MELD) in HCC and LC groups (Table 3).
In HCC cases, no significant difference was observed between IGF-1 levels among different stages of different staging systems (Table 4).
3.4. Serum IGF-1 Predictive Value
The receiver operating characteristic (ROC) curve was used to demonstrate the diagnostic accuracy of IGF-1and AFP in the discrimination between liver cirrhosis and HCC and to determine the cut-off value for IGF-1 for predicting the development of HCC in HBV positive patients with liver cirrhosis. These data show that IGF-1 levels had an area under the ROC curve (AUC) value of 0.891, indicating a high discriminative power between HCC and cirrhosis subsequent promising clinical value. In this study, IGF-1 is proposed as a marker for predicting the development of HCC at an optimal cut-off value of ≤40 with 87.36% sensitivity and 78.16% specificity. When compared with the AFP ROC curve in discrimination between liver cirrhosis and HCC, serum IGF-1 level is a significantly more effective predictor than AFP for the development of HCC in HBV positive patients with cirrhosis with higher sensitivity and specificity.
| - |
Group I HCC |
Group II LC |
Group III CHB |
|||
|---|---|---|---|---|---|---|
| Variables | r | p value | r | p value | r | p value |
| Age | 0.096 | 0.375 | -0.048 | 0.6608 | 0.000 | 0.995 |
| Bilirubin | -0.057 | 0.603 | 0.047 | 0.663 | 0.178 | 0.099 |
| AST | -0.025 | 0.817 | 0.161 | 0.137 | 0.033 | 0.765 |
| ALT | 0.177 | 0.101 | 0.132 | 0.222 | -0.027 | 0.805 |
| Albumin | -0.023 | 0.83 | -0.115 | 0.287 | -0.0421 | 0.699 |
| AFP | -0.087 | 0.423 | 0.022 | 0.837 | 0.186 | 0.085 |
| HBV DNA | -0.095 | 0.382 | -0.19 | 0.08 | 0.07 | 0.522 |
| BMI | -0.108 | 0.321 | -0.035 | 0.625 | -0.04 | 0.714 |
| Hb | 0.072 | 0.51 | -0.093 | 0.394 | -0.141 | 0.192 |
| Creatinine | 0.201 | 0.063 | 0.018 | 0.865 | -0.14 | 0.197 |
| INR | 0.007 | 0.948 | -0.104 | 0.336 | -0.118 | 0.277 |
| MELD | 0.075 | 0.493 | 0.02 | 0.862 | - | - |
| U MELD | 0.055 | 0.614 | 0.022 | 0.84 | - | - |
| WBC | - 0.024 | 0.824 | -0.05 | 0.65 | 0.032 | 0.772 |
| Platelet | 0.139 | 0.201 | -0.084 | 0.442 | -0.16 | 0.139 |
| - | Patients (n = 87) | IGF-1 (ng/ml) Mean ±SD | P-value |
|---|---|---|---|
|
Okuda stage 1 2 3 |
13 33 41 |
33.6 ± 7.1 32.2± 8.7 31.5± 10.3 |
0.775 |
|
CLIP stage 1 2 3 |
9 46 32 |
37.4± 6.8 31± 8.1 32.1± 10.9 |
0.163 |
|
VISUM-HCC 1 2 3 |
41 28 18 |
32.7± 8.7 31.3± 6 31.9± 13.7 |
0.806 |
| Variable/ prediction |
Optimal Cut-off |
Sensitivity (%) |
Specificity (%) |
AUC | 95% Confidence Interval | P-value |
|---|---|---|---|---|---|---|
| IGF-1 / LC | ≤55 | 66.67 | 70.11 | 0.721 | - | - |
| IGF-1/ HCC | ≤40 | 87.36 | 78.16 | 0.891 | 0.199 - 0.391 | < 0.0001 |
| AFP/ HCC | ≥35 | 57.47 | 71.26 | 0.595 |
ROC curve was also used to demonstrate the diagnostic accuracy of IGF-1 to discriminate between HBV positive patients with liver cirrhosis (LC) and CHB patients without cirrhosis where AUC was 0.721 with good discrimination between the 2 groups at a cut-off value of ≤50 with 66.67% sensitivity and 70.11% specificity (Table 5).
4. DISCUSSION
IGF-1 level has been widely investigated as a prognostic factor for the progression and survival of HCC patients with different underlying causes for HCC such as HCV infection, HBV infection, and alcoholic liver cirrhosis [24]. It has been noted in different studies that chronic liver disease decreased serum level and tissue expression of IGF1 and different variations in insulin-like growth factor system components from the start of preneoplastic changes up to the established HCC [25]. It is thought to be a useful marker of liver function, independently of etiology and patient age [26, 27]. So it has been proposed and assessed as a prognostic parameter to assess hepatic reserve in hepatocellular carcinoma [18, 19, 22, 28-30].
Due to their high prevalence in Egypt, HCV and parasitic liver infestations attracted the main focus of publications, whereas HBV related publications represent only 4.2% of the total publications in Egypt [31]. After the literature review, we noticed that most of the studies conducted on the insulin-like growth factor system in Egypt were carried out on HCV positive patients [32-35].
Several studies have reported higher plasma IGF-1 levels in different malignancies such as colorectal, lung, prostate, and breast cancers [15, 17, 36, 37]. In contrast, plasma levels of IGF-1 are decreased in patients with chronic liver disease as it is synthesized in the liver [18]. Another cause for such a decrease in IGF-1 level is the reduction or absence of growth hormone receptors on cirrhotic and HCC cells compared with normal liver tissue [22]. In our study, measurement of serum IGF-1 concentration in the four study groups showed a highly significant reduction in the levels of serum IGF-1 with the progression of HBV infection from chronic infection to liver cirrhosis than HCC. These findings confirmed the previous studies that observed such a decrease in serum IGF-1 with the progression of liver disease. In a previous study where HBV infection was the most common cause of underlying liver disease, the serum IGF-1 level was found to be useful as an indicator of liver function and as a prognostic marker for time to progression and overall survival [38]. A similar association between the liver status and serum IGF-1 level was observed in studies conducted on HCV positive patients that reported a significant reduction of IGF-1 in HCC patients in comparison to patients with cirrhosis [32] and healthy subjects [21]. Also, the concentration of serum IGF-1 was proved to be lower in chronic hepatitis C patients compared to healthy controls [39, 40] and significantly decreased in patients with advanced fibrosis [41-46].
In this study, no significant correlations were detected between serum IGF-1 levels on the one hand and age or different biochemical parameters on the other hand. Moreover, no relation was detected between different HCC staging systems and serum IGF-1 levels. This is in agreement with Aleem et al., who detected no correlation between AFP levels and the levels of IGF-1 in liver cirrhosis and HCC patients as well as the HCC stage [32]. In partial agreement with our findings, the results of Amer et al. have not detected any correlations between IGF-1 and AST, ALT, PT, or bilirubin levels, but there was a significant positive correlation with serum albumin [34]. Discordant with our results, previous studies reported a significant correlation between IGF-1 and various parameters and clinical stages of liver cirrhosis and HCC [38, 40]. This variability in the correlation between IGF-1 level and other parameters may be due to other factors that may govern the production of IGF-1 in chronic liver diseases such as the underlying infection. This is supported by the observed marked decrease in IGF-1 levels in patients infected with HCV rather than HBV [21, 24].
Since 40% of HCC had normal AFP levels [40] and multiple difficulties associated with its use as a biomarker for HCC [41], the use of AFP as a diagnostic marker in HCC is challenging. Roc curve analysis in comparison with AFP revealed that IGF-1 is efficient in discrimination between chronic infection, liver cirrhosis and HCC in HBV infected patients, so it may be a promising marker for the prediction of cirrhosis and HCC development in HBV infected patients. Similar findings were reported in HCC patients infected with HCV where IGF-1 alone showed an AUC of 0.78 that increased to 0.93 in combination with IGFBP-3 for the prediction of HCC development in HCV-infected patients with cirrhosis [32].
CONCLUSION
In conclusion, our results have shown that serum IGF-1 levels are significantly reduced with the progression of hepatic disease in HBV patients, and it may be a promising serological marker alone or in association with others for predicting the development of liver cirrhosis and HCC in chronic HBV patients. Further studies should be directed towards understanding the growth factor levels and expressions in association with HBV.
AUTHORS' CONTRIBUTIONS
Authorship statement: All the authors participated sufficiently in the work and approved the final version of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research was approved by the Ethical Committee of Faculty of Medicine Research, Tanta University, Egypt with approval number 32927/03/18.
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. All human procedures were followed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
CONSENT FOR PUBLICATION
Informed written consent was taken from each patient.
AVAILABILITY OF DATA AND MATERIALS
The authors’ institution does not allow public data access.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors acknowledge all patients participated in this study and take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.


