All published articles of this journal are available on ScienceDirect.
Serum Markers as a Predictor of Hepatic Fibrosis Compared to Fibroscan in chronic hepatitis B Infected Egyptian patients: A Cross-sectional Study
Abstract
Background & Aims:
The gamma-glutamyl transpeptidase (GGT) to platelet ratio (GPR), the gamma-glutamyl transpeptidase to albumin (GAR) and S-index are novel biomarkers suggested to assess liver fibrosis. The aim of the work was to assess the correlation between GGT and other related markers as GAR and GPR among other previous documented markers and the degree of fibrosis and steatosis in chronic HBV Egyptian patients as measured by fibroscan.
Materials And Methods:
After ethical approval of the protocol, a total of 170 chronic HBV patients were recruited from tropical medicine department, Tanta University. They underwent fibroscan examination for fibrosis and steatosis measurement with concomitant testing of liver functions and complete blood picture. Proposed serum markers were calculated. The relation between these ratios with the fibrosis and steatosis measured by fibroscan were tested using Pearson rank correlation.
Results:
There was a highly significant positive correlation between gamma-glutamyl transpeptidase and platelet ratio (GPR), GAR, GGT, Fib4, APRI and fibrosis (p=<0.001, <0.001,<0.001,<0.001,0.011 and <0.001 respectively), while there was no correlation with the degree of steatosis (p=0.922,0.66,0.936,0.214,0.591 and 0.760 respectively). Also these markers were significantly higher in patients with higher grades of fibrosis (f2-4) (p= 0.007,0.013,<0.001,0.018,0.029,and 0.002 respectively), they also showed high sensitivity and low specificity in detecting higher grades of fibrosis with no statistically significant difference between the AUC of GPR and GAR (p=0.89).
Conclusion:
Noninvasive serum markers including GGT, GPR, GAR, Fib4, APRI, and S-index are positively correlated to the degree of fibrosis in CHB patients with high sensitivity and low specificity. They were good negative tests for diagnosis of significant fibrosis.
1. INTRODUCTION
Hepatitis B virus (HBV) infection is a challenging health problem. According to the World Health Organization, an estimated 240 million individuals (3.7%) suffered from chronic HBV infection worldwide [1]. Fibrosis staging is an essential
step in the clinical assessment of patients with chronic HBV (CHB) infection to identify those who require treatment. Liver Biopsy (LB) is an invasive and expensive procedure that is very difficult to perform in routine practice. Thus, non-invasive methods to evaluate liver fibrosis are essential [2-6].
Transient elastography (FibroScan) is considered as a promising noninvasive rapid method for the diagnosis and quantification of hepatic fibrosis in patients with chronic liver disease [2].
Gamma-glutamyl transpeptidase (GGT) is a microsomal enzyme that can be isolated from hepatocytes and gall bladder epithelium. GGT values increase in various liver, gall bladder, and pancreatic diseases [7]. Historically, serum GGT level was a common indicator for hepatobiliary disease reflecting bile duct damage, the progression of liver cirrhosis and chronic hepatitis [8]. In 2016, Lemoine and colleagues presented a simple marker of liver fibrosis depending on the gamma-glutamyl transpeptidase and platelet ratio (GPR) as a marker for diagnosing liver fibrosis in patients with chronic (HBV) infection in West Africa [9]. While another study presented GGT to albumin ratio (GAR) as another marker for diagnosing liver fibrosis in CHB [10].
In addition, S-index that consists of the most significant predictors of fibrosis among routine markers (GGT, PLT and ALB) was simplified from three complicated regression functions; and it had been found to allow identification of both significant fibrosis and cirrhosis using one simple formula [11].
The aim of the study was to assess the accuracy of GGT, its related markers (GPR, GAR, and S-index) and other indirect serum markers versus fibroscan in assessing the degree and stage of fibrosis in chronic HBV Egyptian patients.
2. PATIENTS AND METHODS
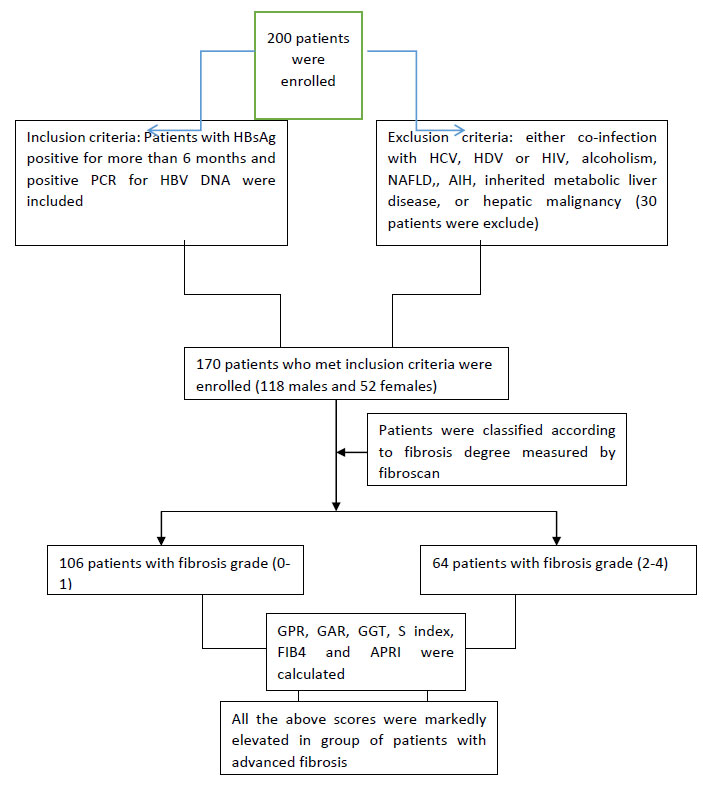
A total of 200 patients were screened for participation in this cross sectional study. Thirty patients were excluded and 170 patients who met the inclusion criteria and presented to the Department of Tropical Medicine and Infectious diseases Tanta University Hospital in the period between December 2018 to June 2019” were enrolled. Ethical committee’s approval with adherence to Helsinki declaration was taken before the start of the study. The aim of the research was made clear to all participants and an informed consent was signed by every patient before enrolment in the study.
All authors had access to the study data and reviewed and approved the final manuscript. A full history was taken from all the patients who fulfilled inclusion criteria of being with chronic hepatitis B with positive PCR for HBV DNA. CHB was defined as the persistent presence of hepatitis B surface antigen (HBsAg) for more than 6 months [12].
Patients with any of the following conditions: infection with hepatitis C virus (HCV), hepatitis D virus (HDV) or human immunodeficiency virus (HIV), significant alcohol consumption (>20 g/day), accompanied by nonalcoholic fatty liver disease (NAFLD), autoimmune liver diseases, inherited metabolic liver disease, or proved hepatic malignancy were excluded from our study.
They were subjected to a full clinical examination, routine laboratory investigation included: CBC, blood urea, serum creatine, ALT, AST, INR, total bilirubin, serum albumin, GGT (Table 1). Serology for hepatitis B surface antigen was detected with an automated blood analyzer (Advia-Bayer, Leverkusen, Germany) [13-19].
Fibroscan Transient Elastography: It was performed on all patients using Echosens™ FibroScan. Fibroscan® probe consists of a 3.5 MHz ultrasound transducer installed on the axis of a low amplitude vibrator (frequency of 50 Hz and amplitude of 2 mm peak to-peak). Liver stiffness measurement (LSM) and Controlled Attenuation Parameter (CAP) were performed by an experienced operator who was blinded to the patient’s diagnosis and data. Only results with 10 valid shots and interquartile range IQR/median liver stiffness ratio < 30% were considered reliable. Both LSM and CAP were obtained in the same area of liver parenchyma (between 25 and 65mm in depth) [2]. The final LSM values and CAP values were expressed in Kpa and dBm−1, respectively.
Definition: Significant fibrosis was defined as fibrosis stage≥F2, severe fibrosis was defined as fibrosis stage≥F3, and cirrhosis was defined as fibrosis stage=F4, according to the METAVIR scoring system. These definitions represented at least significant fibrosis and influenced the management of the patients in terms of treatment indications [3-14].
2.1. Statistical Analysis
Data was analyzed by SPSS V. 23 (SPSS Inc. Released 2015. IBM SPSS statistics for windows, version 23.0, Armnok, NY: IBM Corp.). Paired t test was used to compare readings of normally distributed paired data, and Wilcoxon test was used to compare readings of not-normally distributed paired data. Student’s t-test is a test of significance used for comparison of quantitative variables between two groups of normally distributed data, while Mann Whitney's test was used for not normally distributed ones. Chi-square test (χ2) was used to study association between qualitative variables. Whenever any of the expected cells were less than five, Fischer’s Exact test was used. Logistic regression was performed to ascertain the effect of the significant risk factor of responsiveness to treatment. Receiver Operator Characteristic (ROC) with respective points of maximal accuracy for sensitivity and specificity were generated to determine biomarker performance.
| Models | Formulas |
|---|---|
| AAR | AST (IU/L)/ALT (IU/L) |
| GPR | GGT (IU/L)/PLT (IU/L) |
| GAR | GGT (IU/L)/ALB |
| S index | 1000 × GGT (IU/L)/(PLT (109/L) × ALB2 (g/L)) |
| APRI | AST (IU/L)/ULN (IU/L)/PLT (109/L)) × 100 |
| FIB-4 | (age (years) × AST (IU/L))/(PLT (109/L) × ALT1/2 (IU/L)) |
3. RESULTS
In total, 200 patients were screened for study participation out of whom 30 were excluded from the study; who failed to fulfill the inclusion criteria. This cross-sectional study was conducted on 170 patients presenting to the Department of Tropical Medicine and Infectious diseases Tanta University Hospital (Fig. 1). They were 118 males and 52 females with median age 42.75 ± 12.01.demographic and laboratory data are shown in Table 2.
| Variable | Whole group (n=170) Median, IQ range |
Fibrosis 0-1 (n=106) median, IQ range |
Fibrosis grade 2-4 (n=64) median, IQ range |
|---|---|---|---|
| Hb | 13.0, 12.0-14.20 | 13.15, 11.67-15.07 | 12.9, 12.0-14.60 |
| Platelets | 218, 163.0-257.75 | 220, 175.25-275.25 | 208.0, 152.75-244.50 |
| Total leukocytic count | 6700, 5100-8900 | 6600, 5100-8200 | 6700, 5000-8900 |
| Creatinine | 0.90, 0.80-1.00 | 0.90, 0.72-1.01 | 0.90, 0.80-1.00 |
| Blood urea | 22.00,16.0-32.0 | 22.0, 180.0-32.0 | 22.0, 14.0-32.0 |
| FIB 4 | 1.00, 0.68-1.53 | 0.94, 0.59-1.33 | 1.12, 0.75-2.19 |
| Total bilirubin | 0.90, 0.60-1.02 | 0.80, 0.60-1.00 | 0.90, 0.70-1.04 |
| ALT | 26.0, 22.0-36.0 | 25.0, 21.25-32.0 | 32.0, 23.0-39.0 |
| APRI | 0.32, 0.21-0.47 | 0.28, 0.19-0.40 | 0.40, 0.25-0.58 |
| AST | 26.00, 23.0-35.0 | 25.0, 22.0-32.0 | 30.0, 24.0-42.0 |
| AST/ALT | 1.02, 0.81-1.15 | 1.00, 0.81-1.19 | 1.05, 0.77-1.14 |
| S.albumin | 4.31, 4.10-4.70 | 4.30, 4.0-4.77 | 4.36, 4.15-4.60 |
| INR | 1.02, 1.0-1.10 | 1.02, 1.0-1.07 | 1.02, 1.0-1.10 |
| Prothrombine time | 13.90, 13.0-14.0 | 13.70, 12.62-14.75 | 13.95, 13.0-14.0 |
| Cholesterol | 188.0, 147.0-211.0 | 188.0, 149.0-217.0 | 182.0, 136.5-206.50 |
| Triglyceride | 116.0, 91.0-140.0 | 133.0, 95.0-149.0 | 110.0, 75.25, 138.0 |
| HDL | 41.0, 35.0-57.0 | 45.0, 34.0-57.0 | 39.50, 36.50-51.50 |
| LDL | 101.00, 62.0-144.0 | 101.0, 64.0-145.0 | 99.0, 54.50-139.50 |
| Insulin | 21.10, 13.0-38.25 | 21.10, 13.0-27.60 | 20.70, 11.87-26.77 |
| GGT *103 | 25.00, 15.30-38.25 | 21.0, 13.75-34.50 | 32.0, 22.70-50.75 |
| Alb 2 | 18.49, 16.0-22.09 | 18.49, 16.0-22.32 | 18.61, 16.81-21.16 |
| Plt/alb 2 | 4147200.0, 3041837.0-5054535 | 4329640.0-3167625.0-5315280.0 | 3765960.0, 2710730-4455935 |
| S index | 0.0060, 0.003-0.01 | 0.005, 0.003-0.008 | 0.008, 0.004-0.014 |
| Neutrophils | 55.00, 49.67-60.15 | 55.0, 53.0-57.60 | 55.0, 477.25-64.50 |
| Lymphocytes | 37.60, 31.22-41.0 | 37.20-35.0-41.0 | 38.0, 27.50-40.50 |
| Monocytes | 6.00, 5.0-7.0 | 6.0, 4.75-7.0 | 7.0, 6.0-8.0 |
| GPR | 0.12, 0.06-0.20 | 0.09, 0.05-0.16 | 0.16, 0.11-0.27 |
| GAR | 0.50, 3.33-9.14 | 4.66, 3.01-7.85 | 7.16, 4.70-12.88 |
| Steatosis | 238.0, 201.75-291.50 | 225.0, 205.25-281.75 | 243.0, 196.0-309.0 |
| Fibrosis | 6.05, 4.60-8.67 | 4.90, 4.0-5.85 | 10.30, 7.90-13.0 |
The patients were classified according to the degree of fibrosis to four grades. Patients with significant fibrosis were defined as LS value equal or more than 9kpa (fibrosis grade 2). There were 64 patients (37.6%) with significant fibrosis. Except for AST\ ALT ratio, there was a significant positive correlation found between each of GPR, GAR, GGT, S index, fib 4 and APRI with the grade of fibrosis, while, Steatosis did not have any significant correlation with any of the same markers (Tables 3-5).
| Markers | Grades of Fibrosis | Steatosis | ||
|---|---|---|---|---|
| R | P value | r | P value | |
| GPR | 0.363 | <0.001 | 0.008 | 0.922 |
| GAR | 0.345 | <0.001 | -0.034 | 0.66 |
| GGT | 0.342 | <0.001 | 0.006 | 0.936 |
| S index | 0.337 | <0.001 | -0.096 | 0.214 |
| Fib 4 | 0.197 | 0.011 | 0.042 | 0.591 |
| APRI | 0.274 | <0.001 | 0.024 | 0.760 |
| AST/ALT | 0.023 | 0.768 | 0.037 | 0.632 |
| Markers | Fibrosis grades | P value | |
|---|---|---|---|
| 0-1 (n=106) Median, IQ range |
2-4 (n=64) Median, IQ range |
||
| GGT | 21.0, 13.75-34.50 | 32.0-22.70-50.75 | <0.001 |
| GPR | 0.095, 0.054-0.169 | 0.163, 0.115- 0.271 | 0.007 |
| GAR | 4.66, 3.01-7.85 | 7.163, 4.70-12.88 | 0.013 |
| S index | 0.005, 0.003-0.008 | 0.008, 0.004-0.14 | 0.018 |
| Fib 4 | 0.94, 0.59-1.33 | 1.12, 0.75-2.19 | 0.029 |
| APRI | 0.28, 0.19-0.40 | 0.40, 0.25-0.58 | 0.002 |
| AST/ALT | 1.00, 0.81-1.19 | 1.05, 0.77-1.14 | 0.918 |
| Fibrosis | Cutoff | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|
| GGT | 22.80 | 75.0 | 56.6 | 51 | 79 | 63 |
| GPR | 0.115 | 75.0 | 58.5 | 52 | 79 | 65 |
| GAR | 0.468 | 76.7 | 51.9 | 48 | 79 | 61 |
| S index | 0.0045 | 75.0 | 45.3 | 44 | 75 | 56 |
| Fib 4 | 0.740 | 80.6 | 36.5 | 43 | 76 | 53 |
| APRI | 0.256 | 75.0 | 37.7 | 42 | 71 | 52 |
| AST/ALT | 0.800 | 75.0 | 24.5 | 38 | 62 | 44 |
| Fibrosis | AUC | 95% CI | C st. | |
|---|---|---|---|---|
| Lower | Upper | |||
| GGT | 0.663 | 0.577 | 0.749 | |
| GPR | 0.675 | 0.590 | 0.760 | 0.423 |
| GAR | 0.665 | 0.578 | 0.752 | 0.487 |
| S index | 0.654 | 0.566 | 0.742 | 0.442 |
| Fib 4 | 0.601 | 0.512 | 0.690 | 0.163 |
| APRI | 0.642 | 0.553 | 0.732 | 0.368 |
| AST/ALT | 0.505 | 0.416 | 0.593 | 0.045 |
4. DISCUSSION
Despite that liver biopsy is the main method to determine histological activity and fibrosis level, the need for noninvasive tests to determine fibrosis is increasing due to the rapid development of diagnosis methods and treatment options that may be needed even after transplantation in CHB patients [20-22].
Fibroscan is validated as a method for estimating liver stiffness in different countries [23-27]. But, its performance is limited in several situations including obesity, food intake, ascites and narrow intercostals space [28-30]. In addition, the availability of Fibroscan devices in Egypt is still limited raising the need for alternative noninvasive methods for assessment of hepatic fibrosis.
Multiple noninvasive markers of hepatic fibrosis had been introduced as a substitute to liver biopsy in the last few years [21-30].
In this study we calculated GPR, GAR, GGT, S index, Fib 4, APRI and AST/ALT ratio in CHB and compared them with steatosis and stiffness measures obtained by Fibroscan in order to assess their value as noninvasive markers of fibrosis and steatosis in CHB Egyptian patients.
Except for ALT/AST ratio other markers (GPR, GAR, GGT, S index, Fib 4 and APRI scores) had a significant positive correlation with stiffness measured by Fibroscan. In accordance to our results, Vardar et al 2009 [31] concluded that GGT among other markers was significantly associated with the degree of hepatic fibrosis but they added that it still can't replace liver biopsy.
Meanwhile, Lemoine et al. 2016 [9] compared the accuracy of routine tests as GPR, APRI and Fib-4 to stage liver fibrosis in CHP and they concluded that GPR was the most accurate among these tests in detecting liver fibrosis and that it was a simple and less expensive alternative to liver biopsy and Fibroscan but they had a limitation of small number of their study group. In addition, Hu et al. 2017 [32] had concluded that GPR, APRI, and FIB-4 were positively correlated with hepatic fibrosis.
When we grouped our patients according to stiffness degree measured by fibroscan GPR, GAR, GGT, FIB4, APRI and S-index were found to be higher in patients with significant fibrosis than patients with lower degrees of fibrosis. While AST/ALT ratio did not show any difference between studied groups.
In accordance to our results Eminler et al. 2014 [22] who made liver biopsy to HCV and HBV patients and compared GGT level between different fibrosis grades and they concluded that GGT was higher in patients with higher fibrosis grade especially in HBV patients and GGT should be taken into consideration in diagnosing significant fibrosis, in addition they found a positive correlation between GGT and degree of activity in liver biopsy.
For the estimation of value of these tests in identifying hepatic fibrosis ROC curve analysis was done and we found that these tests had a high sensitivity in detecting significant liver fibrosis (≥f2), unfortunately they had lower specificity making these tests good negative tests in excluding significant liver fibrosis. FIB-4 showed higher sensitivity followed by GAR.
On the contrary to our study, Tarigan and his collegues conducted a study in 2013 [33] on 40 CHB patients and documented higher specificity and sensitivity of S –index (100% and 87.5% respectively) in detecting liver fibrosis and that S-index was more accurate in predicting significant fibrosis and cirrhosis in patients with CHB than APRI but this difference may be due to limited number of patients in their study and different cutoff values. In addition, Zhou et al in 2010 [34] demonstrated that S-index had 42.65% sensitivity and 94.87% specificity in detecting significant fibrosis.
Study limitations: Small sample size, also liver biopsy, which is considered as the gold standard in detecting grade of liver biopsy was not performed. In addition, no control group was included in our study, so additional studies are needed to validate our results.
CONCLUSION
In conclusion we thought that noninvasive serum markers including GGT, GPR, GAR, Fib4, APRI, and S-index are positively correlated to the degree of fibrosis in CHB patients and they are good indicators for exclusion of significant fibrosis. Further studies on larger cohort of patients are needed to validate our results.
AUTHORSHIP STATEMENT
All the authors participated sufficiently in the work and approved the final version of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethical Committee of Faculty of Medicine, Tanta University, Tanta, Egypt, (approval number 30118/05/31).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All patients participated on a voluntary basis and gave their informed consent.
AVAILABILITY OF DATA AND MATERIALS
The data sets used and/or analysed during the current study are available from the corresponding author (S.A.E) on reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


